SEC61B regulates calcium flux and platelet hyperreactivity in diabetes
- PMID: 40829182
- PMCID: PMC12352904
- DOI: 10.1172/JCI184597
SEC61B regulates calcium flux and platelet hyperreactivity in diabetes
Abstract
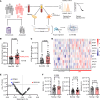
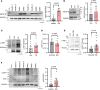
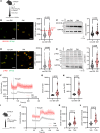
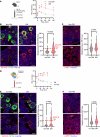
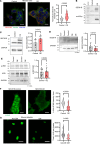
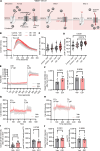
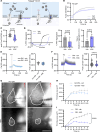
Platelet hyperreactivity increases the risk of cardiovascular thrombosis in diabetes and failure of antiplatelet drug therapies. Elevated basal and agonist-induced calcium flux is a fundamental cause of platelet hyperreactivity in diabetes; however, the mechanisms responsible for this remain largely unknown. Using a high-sensitivity, unbiased proteomic platform, we consistently detected over 2,400 intracellular proteins and identified proteins that were differentially released by platelets in type 2 diabetes. We identified that SEC61 translocon subunit β (SEC61B) was increased in platelets from humans and mice with hyperglycemia and in megakaryocytes from mice with hyperglycemia. SEC61 is known to act as an endoplasmic reticulum (ER) calcium leak channel in nucleated cells. Using HEK293 cells, we showed that SEC61B overexpression increased calcium flux into the cytosol and decreased protein synthesis. Concordantly, platelets in hyperglycemic mice mobilized more calcium and had decreased protein synthesis. Platelets in both humans and mice with hyperglycemia had increased ER stress. ER stress induced the expression of platelet SEC61B and increased cytosolic calcium. Inhibition of SEC61 with anisomycin decreased platelet calcium flux and inhibited platelet aggregation in vitro and in vivo. These studies demonstrate the existence of a mechanism whereby ER stress-induced upregulation of platelet SEC61B leads to increased cytosolic calcium, potentially contributing to platelet hyperreactivity in diabetes.
Keywords: Calcium channels; Cardiology; Cell biology; Hematology; Platelets; Proteomics.
Figures

Comment in
- Chronic diseases alter the platelet rheostat to promote hyperreactivity and thrombosis
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

