Efficacy and safety of anrikefon in patients with pruritus undergoing haemodialysis: multicentre, double blind, randomised placebo controlled phase 3 trial
- PMID: 40829896
- PMCID: PMC12362199
- DOI: 10.1136/bmj-2025-085208
Efficacy and safety of anrikefon in patients with pruritus undergoing haemodialysis: multicentre, double blind, randomised placebo controlled phase 3 trial
Abstract
Objective: To evaluate the efficacy and safety of anrikefon (formerly known as HSK21542), a novel selective peripherally restricted kappa opioid receptor agonist, in patients with chronic kidney disease associated pruritus.
Design: Multicentre, double blind, randomised placebo controlled phase 3 trial.
Setting: 50 centres in China, June 2022 to June 2024.
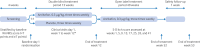
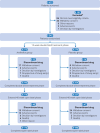
Participants: 652 patients with moderate to severe CKD associated pruritus undergoing haemodialysis were screened: 545 were randomly assigned (1:1 ratio) to receive either anrikefon (n=275) or placebo (n=270).
Interventions: Intravenous anrikefon (0.3 μg/kg body weight) or placebo three times weekly for 12 weeks, followed by an optional open label extension phase with anrikefon treatment for 40 weeks.
Main outcome measures: The primary endpoint was the percentage of patients achieving at least a 4 point reduction in weekly mean 24 hour worst itching intensity numerical rating scale (WI-NRS) score from baseline to week 12. Secondary outcomes were the percentage of patients achieving at least a 3 point reduction in weekly mean WI-NRS score from baseline to week 12, as well as changes in itch related quality of life from baseline using the Skindex-10 and 5-D itch scales. The change in itch related quality of life from baseline to week 40 of open label treatment was also reported using the 5-D itch scale. The safety of anrikefon was evaluated throughout the trial.
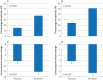
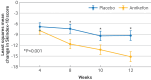
Results: 243/275 (88%) patients in the anrikefon group and 254/270 (94%) in the placebo group completed the 12 week double blind treatment. 443 subsequently entered the 40 week open label extension phase. 37% of patients in the anrikefon group showed at least a 4 point reduction in WI-NRS score at week 12 compared with 15% in the placebo group (P<0.001). The percentage of patients with at least a 3 point reduction in WI-NRS score from baseline to week 12 was 51% in the anrikefon group compared with 24% in the placebo group (P<0.001). The anrikefon group showed significant improvements in itch related quality of life (mean change from baseline in 5-D itch scale -5.3 v -3.1, P<0.001 and in Skindex-10 scale -15.2 v -9.3, P<0.001). Anrikefon also showed sustained long term efficacy during the open label extension phase at week 40, with persistent improvement in quality of life scores on the 5-D itch scale. Mild to moderate dizziness was more common in the anrikefon group than placebo group but without evident clinical consequences.
Conclusions: In patients with moderate to severe pruritus undergoing haemodialysis, anrikefon was found to be safe and resulted in a noticeable reduction in itch intensity and an improvement in itch related quality of life.
Trial registration: ClinicalTrials.gov NCT05135390.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from Haisco Pharmaceutical Group; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
