HPV16 E6 and E7 expressing cancer cells suppress the antitumor immune response by upregulating KLF2-mediated IL-23 expression in macrophages
- PMID: 40829900
- PMCID: PMC12366621
- DOI: 10.1136/jitc-2025-011915
HPV16 E6 and E7 expressing cancer cells suppress the antitumor immune response by upregulating KLF2-mediated IL-23 expression in macrophages
Erratum in
-
Correction: HPV16 E6 and E7 expressing cancer cells suppress the antitumor immune response by upregulating KLF2-mediated IL-23 expression in macrophages.J Immunother Cancer. 2025 Sep 21;13(9):e011915corr1. doi: 10.1136/jitc-2025-011915corr1. J Immunother Cancer. 2025. PMID: 40983345 Free PMC article. No abstract available.
Abstract
Background: Human papillomavirus type 16 (HPV16) positive cancers have a tumor environment that induces antigen-presenting cells to increase IL-23 expression. Unclear is if HPV16 E6/E7 oncoproteins expressed in these cancers play a role in upregulating interleukin (IL)-23 in the tumor microenvironment (TME), and how this cytokine impacts the antitumor cytotoxic T-cell response in HPV16+ cancer.
Methods: CD8 T-cells targeting HPV16+ cancer cells were isolated from C57BL/6 mice bearing HPV16+ C3.43 tumors that were therapeutically vaccinated against HPV16 E6/E7 and incubated with IL-23. These T-cells were then co-incubated with HPV16+ target cells in a cytotoxicity assay to assess their cytolytic capacity. Additionally, carboxyfluorescein succinimidyl ester (CFSE) labeled T-cells were used to track the effect of IL-23 on their proliferation. The effect of IL-23 neutralization on vaccine-induced antitumor immunity during tumor progression was studied in vivo to assess its potential as either a standalone treatment or combined with a vaccine targeting HPV16 E6/E7. HPV16- tumors were engineered to express E6/E7 to find out if these oncoproteins upregulate IL-23. To understand how HPV oncoproteins in the TME affect transcriptional regulation of IL-23 producing cells, we used single-cell Assay for Transposase-Accessible Chromatin (ATAC)+RNA sequencing.
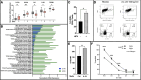
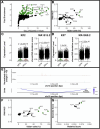
Results: Inside macrophages residing in the HPV+ TME, transcription factor enrichment and linkage analysis identified KLF2 as a potential regulator of Il23a. Overexpression of KLF2 in macrophages upregulates IL-23 production. CD8 T-cells that recognize HPV16+ cells incubated with IL-23 are inhibited in both their killing and proliferative capacities. IL-23 neutralization increased the presence of HPV-specific cytotoxic CD8 T-cells inside the HPV16+TME in an IL-17 independent manner. Combination of IL-23 neutralization followed by HPV16 E6/E7 vaccination increases survival by amplifying the anti-tumor immune response.
Conclusion: This study finds that the presence of HPV oncoproteins in tumor cells increases KLF2 expression in tumor-associated macrophages in vivo. It also shows that KLF2 upregulates IL-23 production in M2 macrophages, resulting in increased IL-23 levels in the TME. In addition, it is shown that elevated levels of IL-23 suppress the antitumor immune response and that IL-23 neutralization synergizes with therapeutic vaccination against HPV oncoproteins.
Keywords: Cervical Cancer; Head and Neck Cancer; Immunosuppression; Immunotherapy; T cell.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
