Pharmacological and structural characterization of vibostolimab, a novel anti-TIGIT blocking antibody for cancer immunotherapy
- PMID: 40829901
- PMCID: PMC12366625
- DOI: 10.1136/jitc-2024-008972
Pharmacological and structural characterization of vibostolimab, a novel anti-TIGIT blocking antibody for cancer immunotherapy
Abstract
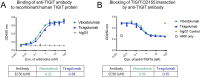
Background: Vibostolimab is a humanized anti-T cell immunoreceptor with immunoglobulin (Ig) and ITIM domains (TIGIT) antibody was recently investigated in late-stage clinical trials. Vibostolimab was developed based on its specific binding property to the human and cynomolgus monkey TIGIT protein and its functional blocking activity of the TIGIT:CD155 interaction.
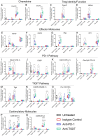
Methods: The biochemical properties of vibostolimab, in a comparison with tiragolumab, were assessed with Biacore, nuclear magnetic resonance (NMR), and X-ray crystallography, while the T-cell activation was examined using engineered Jurkat cells in an in vitro co-culture system by measuring interleukin (IL)-2 production as a readout of T-cell activation. The mouse surrogate anti-TIGIT antibodies were identified and used to extensively investigate the mechanism of action of anti-TIGIT antibodies as monotherapy or in combination with anti-programmed cell death protein-1 (PD-1) antibody in vivo by gene expression profiling and flow cytometry analyses with a mouse syngeneic tumor model.
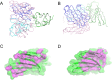
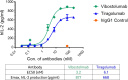
Results: Structural analyses with solution NMR and X-ray crystallography revealed that vibostolimab binds the CC'C''FG loop interface of TIGIT, completely competing for the binding site of CD155 with a larger coverage area than another anti-TIGIT antibody tiragolumab, consistent with surface plasmon resonance analysis, though mouse models with similar antibodies revealed no functional difference in antitumor efficacy. Biacore-based binding data indicate that both vibostolimab and tiragolumab bind on TIGIT with comparable binding affinity (KD), but different binding kinetics characterized by faster on and off rates, which may contribute to enhanced T-cell activation with vibostolimab, as evidenced by greater IL-2 production in an in vitro cell-based assay. Functional experiments in mouse models demonstrated additional detailed biological mechanisms of the mouse equivalent of vibostolimab, including the differentiating role of myeloid cell activation in addition to improved T cell cytolytic activity, both of which are further enhanced by anti-PD-1 combination therapy.
Conclusions: Vibostolimab is a novel anti-TIGIT antibody that completely blocks CD155 binding and induces T-cell activation. From experiments using a mouse tumor model and mouse surrogate anti-TIGIT antibody, we demonstrate direct evidence where it not only activates cytotoxic T cells but also induces the activation of antigen-presenting cells. The clinical relevance of vibostolimab, based on these mechanisms, in combination with pembrolizumab was recently tested in registrational trials.
Keywords: Immune Checkpoint Inhibitor; Immunotherapy; Monoclonal antibody; T cell.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: All authors are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Rahway, New Jersey, USA and shareholders of Merck & Co, Rahway, New Jersey, USA.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
