HIIT and MICT mitigate endothelial dysfunction in early atherosclerotic mice via PCSK9 inhibition
- PMID: 40830352
- PMCID: PMC12365230
- DOI: 10.1038/s41598-025-05206-7
HIIT and MICT mitigate endothelial dysfunction in early atherosclerotic mice via PCSK9 inhibition
Abstract
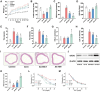
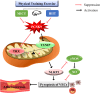
Atherosclerosis (AS), driven by vascular endothelial dysfunction and poses a global health threat. This study compared the therapeutic effects of high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT) on vascular endothelial function in early-stage AS mice, specifically investigating PCSK9 modulation and the TRX/TXNIP/NLRP3/GSDMD-N pathway. ApoE-/- mice (n = 6/group) fed a high-fat diet for 12 weeks were randomized into sedentary (AS-S), HIIT (AS-HIIT), and MICT (AS-MICT) groups, with wild-type mice as control. Training lasted 12 weeks. Outcomes included body weight, lipid profiles (TG, TC, LDL-C, HDL-C), oxidative stress markers (T-SOD, GSH-Px, MDA), vascular function (eNOS expression, ACh-induced vasorelaxation), and TRX/TXNIP/NLRP3/GSDMD-N pathway activity. Both HIIT and MICT reduced body weight (p < 0.05) and improved lipid profile. Exercise groups showed reduced oxidative stress and inflammation pathways (p < 0.05). HIIT and MICT ameliorate early AS by reducing PCSK9 and oxidative/inflammatory pathway levels (p < 0.05), but HIIT demonstrates superior efficacy in improving endothelial function and pathway activation. These findings show HIIT and MICT mitigate endothelial dysfunction in early atherosclerotic mice via PCSK9 inhibition and advocate for HIIT as a prioritized strategy in early AS management.
Keywords: Atherosclerosis; Endothelial dysfunction; Exercise; Pyroptosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: All research procedures were approved by the Ethics Committee of Chongqing Medical University (No.2021101) and adhered to the regulations of the People’s Republic of China governing the management of laboratory animals. Informed consent : All authors have read and approved the final version of the manuscript and agreed to the order of authorship.
Figures



Similar articles
-
The impact of high-intensity interval training versus moderate-intensity continuous training on vascular function: a systematic review and meta-analysis.Sports Med. 2015 May;45(5):679-92. doi: 10.1007/s40279-015-0321-z. Sports Med. 2015. PMID: 25771785
-
Effects of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training On Blood Pressure in Adults with Pre- to Established Hypertension: A Systematic Review and Meta-Analysis of Randomized Trials.Sports Med. 2018 Sep;48(9):2127-2142. doi: 10.1007/s40279-018-0944-y. Sports Med. 2018. PMID: 29949110
-
High-Intensity Interval Training Programs Versus Moderate-Intensity Continuous Training for Individuals With Heart Failure: A Systematic Review and Meta-analysis.Arch Phys Med Rehabil. 2025 Jan;106(1):98-112. doi: 10.1016/j.apmr.2024.05.028. Epub 2024 Jun 9. Arch Phys Med Rehabil. 2025. PMID: 38862032
-
A systematic review and meta-analysis of interval training versus moderate-intensity continuous training on body adiposity.Obes Rev. 2017 Aug;18(8):943-964. doi: 10.1111/obr.12536. Epub 2017 May 17. Obes Rev. 2017. PMID: 28513103
-
Effects of high-intensity interval and continuous moderate aerobic training on fitness and health markers of older adults: A systematic review and meta-analysis.Arch Gerontol Geriatr. 2024 Sep;124:105451. doi: 10.1016/j.archger.2024.105451. Epub 2024 Apr 22. Arch Gerontol Geriatr. 2024. PMID: 38718488
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

