Molecular mechanisms of thiazide-like diuretics-mediated inhibition of the human Na-Cl cotransporter
- PMID: 40830368
- PMCID: PMC12365235
- DOI: 10.1038/s41467-025-62714-w
Molecular mechanisms of thiazide-like diuretics-mediated inhibition of the human Na-Cl cotransporter
Abstract
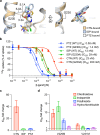
Thiazide-type and thiazide-like diuretics are structurally distinct first-line antihypertensive drugs that target the sodium-chloride cotransporter (NCC) in the kidney. Thiazide-like diuretics are reported to have better cardioprotective effects than thiazide-type diuretics, but whether this is due to differences in NCC-inhibition mechanisms, if there is any, remains unclear. To understand the molecular mechanisms of NCC inhibition by thiazide-like diuretics, we determine the structures of human NCC (hNCC) bound to two of the most widely used thiazide-like diuretics, chlorthalidone and indapamide, using cryogenic electron microscopy (cryo-EM). Structural analyses reveal shared features and distinctions between NCC-inhibition by thiazide-like and thiazide-type diuretics. Furthermore, structural comparisons allow us to identify polymorphisms in hNCC that have substantial differential effects on the potencies of specific thiazide-like and thiazide-type diuretics. Our work provides important insights into the molecular pharmacology of NCC and a blueprint for developing precision medicine to manage hypertension with thiazide-like and thiazide-type diuretics.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



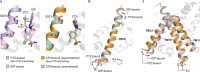

Similar articles
-
Blood pressure-lowering efficacy of monotherapy with thiazide diuretics for primary hypertension.Cochrane Database Syst Rev. 2014 May 29;2014(5):CD003824. doi: 10.1002/14651858.CD003824.pub2. Cochrane Database Syst Rev. 2014. PMID: 24869750 Free PMC article.
-
Transport and thiazide-inhibition mechanisms of the Na-Cl cotransporter: a structural perspective.Curr Opin Nephrol Hypertens. 2025 Sep 1;34(5):440-449. doi: 10.1097/MNH.0000000000001099. Epub 2025 Jul 3. Curr Opin Nephrol Hypertens. 2025. PMID: 40575829 Free PMC article. Review.
-
Structural bases for Na+-Cl- cotransporter inhibition by thiazide diuretic drugs and activation by kinases.Nat Commun. 2024 Aug 14;15(1):7006. doi: 10.1038/s41467-024-51381-y. Nat Commun. 2024. PMID: 39143061 Free PMC article.
-
Structure and thiazide inhibition mechanism of the human Na-Cl cotransporter.Nature. 2023 Feb;614(7949):788-793. doi: 10.1038/s41586-023-05718-0. Epub 2023 Feb 15. Nature. 2023. PMID: 36792826 Free PMC article.
-
Thiazide diuretics alone or in combination with a potassium-sparing diuretic on blood pressure-lowering in patients with primary hypertension: protocol for a systematic review and network meta-analysis.Syst Rev. 2022 Feb 8;11(1):23. doi: 10.1186/s13643-022-01890-y. Syst Rev. 2022. PMID: 35135630 Free PMC article.
References
-
- World Health Organization. Global report on hypertension: The race against a silent killer (World Health Organization, 2023).
-
- Messerli, F. H., Williams, B. & Ritz, E. Essential hypertension. Lancet370, 591–603 (2007). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

