Impact of CAR T cell therapy on thymus size in children and young adults with acute lymphoblastic leukemia
- PMID: 40830378
- PMCID: PMC12365290
- DOI: 10.1038/s41598-025-12630-2
Impact of CAR T cell therapy on thymus size in children and young adults with acute lymphoblastic leukemia
Abstract
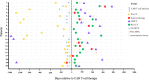
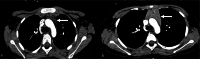
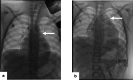
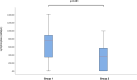
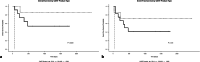
Chimeric Antigen Receptor (CAR) T-cell therapy has demonstrated efficacy in children and young adult patients with acute lymphoblastic leukemia (ALL). The purpose of our study was to investigate thymus size changes after CAR T-cell therapy, explore the associated clinical conditions, and assess survival differences of patients who underwent CAR T-cell therapy, we conducted a single-center retrospective study of children and young adult patients who underwent CAR T-cell therapy for ALL between April 2015 and October 2023.We measured the volume of the thymus on pre- and post-CAR T-cell chest CT scans of 20 patients (median [IQR] age, 18[11] years; 11 females). We divided patients into two groups, those who did (group 1) or did not (group 2) demonstrate increase in thymus size after therapy. Clinical and survival data were collected. We used the Wilcoxon signed-rank test or Fisher's exact test for group comparisons and analyzed event-free survival data. Seven of 20 patients (35%, group 1) showed increase in thymus volume (pre- vs. post-CAR T-cell thymus volume; 5.01 [2.18] cm³ vs. 20.87 [19.86] cm³, p = 0.01), while 13 patients (65%, group 2) showed no increase in thymus volume (pre- vs. post-CAR T-cell thymus volume; 3.01 [13.42] cm³ vs. 2.09 [8.34] cm³, p = 0.01). Patients in group 1 were younger (12 [8] years vs. 19[10] years, p = 0.028) and showed a higher rate of event-free survival compared to those in group 2 (p = 0.003). In children and young adults with ALL, increased thymus size after CAR T-cell therapy was associated with younger age and improved clinical outcomes.
Keywords: CAR T-cell therapy; Chest CT; Child; Event-free survival; Leukemia; Thymus.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Low Peripheral Blood Counts and Elevated Proinflammatory Cytokines Signal a Poor CD19 Chimeric Antigen Receptor T-cell Response in Acute Lymphoblastic Leukemia.Transplant Cell Ther. 2025 Aug;31(8):551-564. doi: 10.1016/j.jtct.2025.05.003. Epub 2025 May 20. Transplant Cell Ther. 2025. PMID: 40398620
-
Dasatinib and CAR T-Cell Therapy in Newly Diagnosed Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Nonrandomized Clinical Trial.JAMA Oncol. 2025 Jun 1;11(6):625-629. doi: 10.1001/jamaoncol.2025.0674. JAMA Oncol. 2025. PMID: 40244598 Free PMC article. Clinical Trial.
-
Post-transplant relapse in pediatric acute lymphoblastic leukemia in the era of CAR-T cell therapy. A multicenter analysis of Grupo Español de Trasplante Hematopoyetico y Terapia Celular (GETH-TC) Pediatric Committee.Cytotherapy. 2025 Apr;27(4):438-445. doi: 10.1016/j.jcyt.2024.11.016. Epub 2024 Dec 2. Cytotherapy. 2025. PMID: 39708044
-
Chimeric antigen receptor (CAR) T-cell therapy for people with relapsed or refractory diffuse large B-cell lymphoma.Cochrane Database Syst Rev. 2021 Sep 13;9(9):CD013365. doi: 10.1002/14651858.CD013365.pub2. Cochrane Database Syst Rev. 2021. PMID: 34515338 Free PMC article.
-
Nutritional interventions for survivors of childhood cancer.Cochrane Database Syst Rev. 2016 Aug 22;2016(8):CD009678. doi: 10.1002/14651858.CD009678.pub2. Cochrane Database Syst Rev. 2016. PMID: 27545902 Free PMC article.
References
-
- Institute NC. SEER cancer stat facts: Childhood leukemia (Ages 0–19). https://seer.cancer.gov/statfacts/html/childleuk.html. (2022).
-
- Siegel, R. L. M. K., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA: A cancer J. Clin.72, 7–33. 10.3322/caac.21708 (2022). - PubMed
-
- John, S. P. M. et al. Real-world outcomes for pediatric and young adult patients with relapsed or refractory (R/R) B-cell acute lymphoblastic leukemia (ALL) treated with tisagenlecleucel: Update from the center for international blood and marrow transplant research (CIBMTR) registry. Blood138(Supplement 1), 428. 10.1182/blood-2021-146393 (2021).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

