The clinical impact of oral iron treatment for anaemia in pregnancy in accordance with current guidance: a prospective cohort study in a maternity unit in the Midlands of England
- PMID: 40830435
- PMCID: PMC12366138
- DOI: 10.1186/s12884-025-07938-w
The clinical impact of oral iron treatment for anaemia in pregnancy in accordance with current guidance: a prospective cohort study in a maternity unit in the Midlands of England
Abstract
Background: Iron deficiency anaemia is a common disorder affecting up to 30% of pregnant women. Treatment guidelines for iron deficiency anaemia in pregnancy exist, which if adopted, may reduce the associated risks of maternal and fetal morbidity and mortality. However, multiple factors may impair adherence and absorption of oral iron, limiting the success of this first-line treatment.
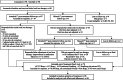
Methods: To document the effectiveness of national (British Society of Haematology) guidelines for the treatment of iron deficiency anaemia (IDA) in pregnancy, with a focus on use of oral iron, we carried out a prospective cohort study. Aims were to assess the response, side effect and adherence to treatment and predictability of response using routine clinical and laboratory data. The study population consisted of pregnant women diagnosed with anaemia. Women were offered follow-up through a dedicated anaemia clinic in a secondary care maternity unit serving a multi-ethnic population in the midlands of England. First line treatment was ferrous sulphate 200 mg three time a day as recommended in earlier national guidelines. The response was assessed 2 to 4 weeks later by measuring the haemoglobin (Hb) concentration. A response was defined in 2 ways; (i) a 10 g/L increase in Hb; and (ii) a 10 g/L increase in Hb and/or gestationally adjusted threshold of the Hb. Education and advice were provided to women, with on-going follow-up at clinic appointments including an assessment of side effects. Following a response with oral iron, treatment was continued for a further 3 months when the women were again reviewed.
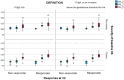
Results: The overall rate of haematological response to a first course of oral iron was 36.5% (10 g/L increase in Hb) and 55.2% (incorporating gestational threshold in Hb). The response rates at the completion of follow up, post-delivery, were 70.5% and 88.5% respectively. Responders to oral iron had lower median Hb at diagnosis (95 g/L) compared to non-responders (100 g/L). The responders median Hb was 113 g/l versus 103 g/L for non-responders at first follow-up and was Hb 122 g/L versus 110 g/L, respectively, at the end of the study visit 5. There is a statistically significant difference between responders and non-responders for the change in haemoglobin from baseline to visit 5 (p = 0.017). Non-responders reported more side effects than responders (95% versus 85%).
Conclusion: Oral iron treatment for IDA in pregnancy as advocated in national guidelines is challenging to deliver, even in the setting of a specialist anaemia clinic. The findings have implications for guideline recommendations and implementation, and identify research opportunities for diagnosing IDA in pregnancy, optimising the pathways of iron treatment.
Keywords: Adherence; Anaemia; Diagnosis; Iron; Prediction; Response.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethics approval was granted by the West Midlands– Black Country Ethics committee. Ref number 18/WM/0090. All participants provided informed consent to participate in the study in writing using the ethically approved consent form. Consent for publication: Not applicable. Competing interests: Competing Interests. D Churchill was formerly a member of the Multi-disciplinary Iron Deficiency Anaemia Steering (MIDAS) committee supported by Pharmacosmos. All other authors do not have any competing interests.
Figures

References
-
- Royal College of Obstetricians and Gynaecologists. Blood Transfusions in Obstetrics. Green-top Guideline No. 47. 2015. Available from: https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guideline...
-
- National Institute for Health and Care Excellence. Antenatal Care. NICE guideline [NG201]. Available from: https://www.nice.org.uk/guidance/ng201
-
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Available from: https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

