Evaluation and interpretation of DTI-ALPS, a proposed surrogate marker for glymphatic clearance, in a large population-based sample
- PMID: 40830522
- PMCID: PMC12362905
- DOI: 10.1186/s13195-025-01842-3
Evaluation and interpretation of DTI-ALPS, a proposed surrogate marker for glymphatic clearance, in a large population-based sample
Abstract
Background: The diffusion tensor imaging along perivascular spaces index (DTI-ALPS), which measures diffusivity in the perivascular spaces along the medullary veins, has gained popularity and controversy as a surrogate marker of glymphatic clearance. The goal of this work is to automatically estimate DTI-ALPS in a large population-based sample, evaluate the correlates of the signal observed in the context of aging and dementia biomarkers, and evaluate its clinical usefulness.
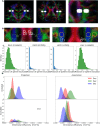
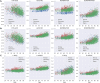
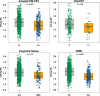
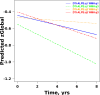
Methods: We identified 2715 participants aged 30 + years in the population-based Mayo Clinic Study of Aging with diffusion MRI. We calculated DTI-ALPS through a modified pipeline of previously published methods. We evaluated DTI-ALPS using different protocols and scanners and reported ICC for agreement. We examined the predictors of longitudinal DTI-ALPS with demographics (age, sex), vascular risk, clinical data (diagnosis, global cognition), and imaging markers (white matter hyperintensity (WMH), global amyloid load from PIB-PET, and temporal meta-ROI Tau-PET SUVR) in a subset of participants aged 50 + years using Pearson correlations, ANCOVA with adjustments for age and sex, and linear mixed effect models. We also compared the utility of DTI-ALPS with WMH for prediction of cognitive decline.
Results: With modifications to the automated DTI-ALPS pipeline, consistent measurements can be made from data obtained with different protocols on different scanners. DTI-ALPS was negatively correlated with age, vascular risk, and WMH burden and was positively correlated with cognitive scores and higher in females. In the longitudinal models, WMH explained the greatest variability in decline of DTI-ALPS. The age and sex adjusted associations with AD biomarkers (amyloid and tau) were minimal. DTI-ALPS had a significant interaction with WMH on the rate of cognitive decline.
Conclusions: DTI-ALPS can be reliably automated in large samples. The computed DTI-ALPS was associated with vascular dysfunction (vascular risk and WMH) and may provide additional complementary information about cognitive decline. The low associations with AD biomarkers suggest that DTI-ALPS may be a poor surrogate of AD.
Keywords: Amyloid deposition; DTI-ALPS; Vascular dysfunction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards (IRB) and written informed consent was obtained from all participants or their surrogates in accordance with the Declaration of Helsinki. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
Glymphatic function associates with Alzheimer's disease-signature region volumes, plasma biomarkers and white matter hyperintensity progression in cognitively unimpaired older adults.Age Ageing. 2025 May 31;54(6):afaf141. doi: 10.1093/ageing/afaf141. Age Ageing. 2025. PMID: 40459343 Free PMC article.
-
MRI free water mediates the association between diffusion tensor image analysis along the perivascular space and executive function in four independent middle to aged cohorts.Alzheimers Dement. 2025 Feb;21(2):e14453. doi: 10.1002/alz.14453. Epub 2024 Dec 30. Alzheimers Dement. 2025. PMID: 39740225 Free PMC article.
-
Diffusion Tensor Imaging-Along the Perivascular-Space Index Is Associated with Disease Progression in Parkinson's Disease.Mov Disord. 2024 Sep;39(9):1504-1513. doi: 10.1002/mds.29908. Epub 2024 Jul 11. Mov Disord. 2024. PMID: 38988232 Free PMC article.
-
Diffusion Tensor Imaging Along the Perivascular Space for Characterizing Cerebral Interstitial Fluid Dynamics in Alzheimer's Disease: A Systematic Review and Meta-Analysis.AJNR Am J Neuroradiol. 2025 Aug 4:ajnr.A8953. doi: 10.3174/ajnr.A8953. Online ahead of print. AJNR Am J Neuroradiol. 2025. PMID: 40759558 Review.
-
Reliability and Spatial Consistency of MR Diffusion Tensor Imaging Measures Along the Cerebral Perivascular Space.J Neuroimaging. 2025 Sep-Oct;35(5):e70086. doi: 10.1111/jon.70086. J Neuroimaging. 2025. PMID: 40903913 Free PMC article. Review.
References
-
- Taoka T, Naganawa S. Glymphatic imaging using MRI. J Magn Reson Imaging. 2020;51(1):11–241053. - PubMed
-
- Zhang W, et al. Glymphatic clearance function in patients with cerebral small vessel disease. NeuroImage. 2021;238:1182571053–8119. - PubMed
-
- Fraum TJ, et al. Gadolinium-based contrast agents: a comprehensive risk assessment. J Magn Reson Imaging. 2017;46(2):338–53. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

