Stabilization of norovirus GII.3 virus-like particles by rational disulfide engineering
- PMID: 40830611
- PMCID: PMC12365219
- DOI: 10.1038/s41541-025-01254-2
Stabilization of norovirus GII.3 virus-like particles by rational disulfide engineering
Abstract
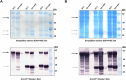
Noroviruses are non-enveloped, single-stranded positive-sense RNA viruses and the leading cause of gastroenteritis worldwide. The major capsid protein, VP1, can self-assemble into non-infectious virus-like particles (VLPs), representing an attractive vaccine platform. It was demonstrated that engineered disulfide bonds within VP1 could significantly stabilize VLPs of the archetypal GI.1 strain. Here, we apply a similar strategy to VLPs of multiple circulating GII genotypes. We find that engineered disulfide mutations can significantly stabilize VLPs of the GII.3 strain, but not the closely related GII.6 strain. Disulfide-stabilized GII.3 VLPs (GII.3-DS1) exhibit increased yields, greater homogeneity, and higher thermal stability compared to wild-type GII.3 VLPs. GII.3-DS1 VLPs are a superior reagent in immunological assays compared to the wild-type counterpart. Importantly, mRNA encoding GII.3-DS1 elicits superior humoral immune responses compared to wild-type GII.3 mRNA in mice. These results demonstrate the utility of rational VLP stabilization for advancing vaccine development efforts.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: All co-authors are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stock or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. This does not alter the authors’ adherence to all journal policies on competing interests and sharing data and materials. In addition, a subset of the authors is listed as inventors on a patent surrounding this work held by Merck & Co., Inc., Rahway, NJ, USA.
Figures



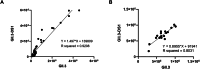

References
LinkOut - more resources
Full Text Sources
Research Materials

