Targeting iron-associated protein Ftl1 in the brain of old mice improves age-related cognitive impairment
- PMID: 40830655
- PMCID: PMC12532579
- DOI: 10.1038/s43587-025-00940-z
Targeting iron-associated protein Ftl1 in the brain of old mice improves age-related cognitive impairment
Abstract
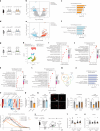
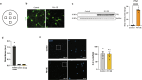
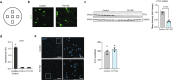
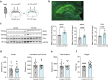
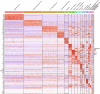
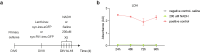
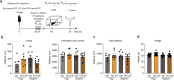
Understanding cellular and molecular drivers of age-related cognitive decline is necessary to identify targets to restore cognition at old age. Here we identify ferritin light chain 1 (FTL1), an iron-associated protein, as a pro-aging neuronal factor that impairs cognition. Using transcriptomic and mass spectrometry approaches, we detect an increase in neuronal FTL1 in the hippocampus of aged mice, the levels of which correlate with cognitive decline. Mimicking an age-related increase in neuronal FTL1 in young mice alters labile iron oxidation states and promotes synaptic and cognitive features of hippocampal aging. Targeting neuronal FTL1 in the hippocampi of aged mice improves synaptic-related molecular changes and cognitive impairments. Using neuronal nuclei RNA sequencing, we detect changes in metabolic processes, such as ATP synthesis, and boosting these metabolic functions through NADH supplementation mitigated pro-aging effects of neuronal FTL1 on cognition. Our data identify neuronal FTL1 as a key molecular mediator of cognitive rejuvenation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: S.A.V. consulted for SV Health Investors and The Herrick Company, Inc. All other authors declare no competing interests.
Figures







References
-
- Bieri, G., Schroer, A. B. & Villeda, S. A. Blood-to-brain communication in aging and rejuvenation. Nat. Neurosci.26, 379–393 (2023). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

