A plasma proteomics-based candidate biomarker panel predictive of amyotrophic lateral sclerosis
- PMID: 40830661
- PMCID: PMC12532604
- DOI: 10.1038/s41591-025-03890-6
A plasma proteomics-based candidate biomarker panel predictive of amyotrophic lateral sclerosis
Abstract
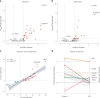
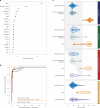
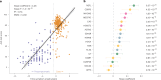
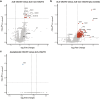
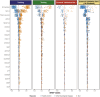
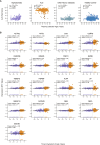
Identifying a reliable biomarker for amyotrophic lateral sclerosis (ALS) is crucial for clinical practice. Here, in this cross-sectional study, we used the Olink Explore 3072 platform to investigate plasma proteomics as a biomarker tool for this neurodegenerative condition. Thirty-three proteins were differentially abundant in the plasma of patients with ALS (n = 183) versus controls (n = 309). We replicated our findings in an independent cohort (n = 48 patients with ALS and n = 75 controls). We then applied machine learning to create a model that diagnosed ALS with high accuracy (area under the curve, 98.3%). By analyzing plasma samples from individuals before ALS symptoms emerged, we estimated the age of clinical onset and showed that the disease process-impacting skeletal muscle, nerves and energy metabolism-occurs years before symptoms appear. Our research suggests that plasma proteins can be a biomarker for this fatal disease and offers molecular insights into its prodromal phase.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: R.C., R.M., J.Y.K., L.F., C.L.D., S.W.S. and B.J.T. have a patent pending (US Patent application no. 63/717,807) on the diagnostic testing for ALS based on the proteomic panel. B.J.T. holds patents on the clinical testing and therapeutic intervention for the hexanucleotide repeat expansion of C9orf72. B.J.T. and S.W.S. receive research support from Cerevel Therapeutics. S.W.S. serves on the scientific advisory committees of the Lewy Body Dementia Association, Mission MSA and the GBA1 Canada Initiative. The other authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

