Bacillus subtilis ED24 Controls Fusarium culmorum in Wheat Through Bioactive Metabolite Secretion and Modulation of Rhizosphere Microbiome
- PMID: 40830705
- PMCID: PMC12364970
- DOI: 10.1007/s00248-025-02590-5
Bacillus subtilis ED24 Controls Fusarium culmorum in Wheat Through Bioactive Metabolite Secretion and Modulation of Rhizosphere Microbiome
Abstract
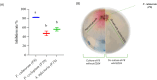
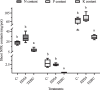
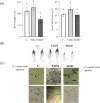
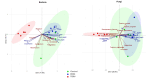
Fusarium culmorum is a soil-borne fungal pathogen causing root and stem rot, seedling blight, and significant yield losses in small grain cereals, including wheat. This study aimed to evaluate the antifungal potential of Bacillus subtilis ED24, an endophytic strain isolated from Ziziphus lotus (L.) roots, and its effects on wheat growth and yield under controlled conditions. In vitro assays demonstrated that B. subtilis ED24 inhibited F. culmorum mycelial growth by up to 87%, associated with the secretion of 37 distinct secondary metabolites, predominantly involved in carbon cycling. In pot experiments, B. subtilis ED24 significantly enhanced wheat germination (85%) and growth compared to infected plants treated with the chemical fungicide tebuconazole. Although nutrient analysis showed significantly higher shoot nitrogen (32.34 mg/pot) and phosphorus (2.41 mg/pot) contents in the B. subtilis ED24 treatment compared to tebuconazole (8.11 and 0.18 mg/pot, respectively), no significant differences were observed when compared to the infected control (C-). Similarly, B. subtilis ED24 led to improved thousand grain weight (40.4 g), protein content (19.98%), and ash content (1.95%) relative to tebuconazole (29.1 g, 18.31%, and 1.74%, respectively), yet these values did not differ significantly from the infected control (C-). Notably, the number of seeds per pot was significantly increased by B. subtilis ED24 compared to the infected control (C-) (113.8 seeds/pot vs. 54.2 seeds/pot). Additionally, B. subtilis ED24 modulated the wheat rhizosphere microbiome, enriching beneficial taxa such as Eurotiomycetes fungal class and the bacterial genus Paramesorhizobium. These findings suggest that the antifungal activity and growth-promoting effects of B. subtilis ED24 are likely mediated through the synthesis of unique bioactive metabolites and microbiome modulation, offering a promising sustainable alternative to chemical fungicides in wheat production.
Keywords: Triticum durum; Ziziphus lotus (L.) Desf.; Endophyte; Propionic acid; Rhizosphere microbiota; Secondary metabolites; Tebuconazole.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Wheat rhizosphere persistence of Trichoderma gamsii A5MH during suppression of a Fusarium-Pythium root disease complex differentially impacts the soil fungal and oomycete microbiome.J Appl Microbiol. 2025 Jul 1;136(7):lxaf158. doi: 10.1093/jambio/lxaf158. J Appl Microbiol. 2025. PMID: 40560537
-
Exploring the agronomic traits, antioxidant and antifungal properties of Hermetia illucens frass extract in durum wheat (Triticum durum Desf.).BMC Plant Biol. 2025 Aug 14;25(1):1075. doi: 10.1186/s12870-025-07086-5. BMC Plant Biol. 2025. PMID: 40813627 Free PMC article.
-
Genomic and Phenotypic Insights into the Potential of Bacillus subtilis YB-15 Isolated from Rhizosphere to Biocontrol against Crown Rot and Promote Growth of Wheat.Biology (Basel). 2022 May 20;11(5):778. doi: 10.3390/biology11050778. Biology (Basel). 2022. PMID: 35625506 Free PMC article.
-
Root architecture and the rhizosphere microbiome: Shaping sustainable agriculture.Plant Sci. 2025 Oct;359:112599. doi: 10.1016/j.plantsci.2025.112599. Epub 2025 Jun 5. Plant Sci. 2025. PMID: 40482721 Review.
-
The chemical interaction between plants and the rhizosphere microbiome.Trends Plant Sci. 2025 Sep;30(9):1002-1019. doi: 10.1016/j.tplants.2025.06.001. Epub 2025 Jul 1. Trends Plant Sci. 2025. PMID: 40603217 Free PMC article. Review.
References
-
- Amein TAM (2023) Differences among three wheat cultivars in susceptibility to Fusarium culmorum and the effect of a number of bacterial strains on infested seed germination. Zanco J Pure Appl Sci 35:113–120. 10.21271/ZJPAS.35.4.11
-
- Haegler P, Joerin L, Krähenbühl S, Bouitbir J (2017) Hepatocellular toxicity of imidazole and triazole antimycotic agents. Toxicol Sci 157:183–195. 10.1093/toxsci/kfx029 - PubMed
-
- Miller SA, Ferreira JP, LeJeune JT (2022) Antimicrobial use and resistance in plant agriculture: a one health perspective. Agriculture 12:289. 10.3390/agriculture12020289
-
- Roman DL, Voiculescu DI, Ostafe V et al (2022) A review of the toxicity of triazole fungicides approved to be used in European Union to the soil and aqueous environment. Ovidius University Annals of Chemistry 33:113–120. 10.2478/auoc-2022-0017
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

