Innovative approaches to combat antibiotic resistance: integrating CRISPR/Cas9 and nanoparticles against biofilm-driven infections
- PMID: 40830872
- PMCID: PMC12366227
- DOI: 10.1186/s12916-025-04323-4
Innovative approaches to combat antibiotic resistance: integrating CRISPR/Cas9 and nanoparticles against biofilm-driven infections
Abstract
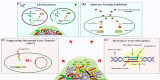
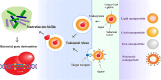
The increasing prevalence of antibiotic-resistant bacterial infections is a major global health concern, with biofilms playing a key role in bacterial persistence and resistance. Biofilms provide a protective matrix that limits antibiotic penetration, enhances horizontal gene transfer, and enables bacterial survival in hostile environments. Conventional antimicrobial therapies are often ineffective against biofilm-associated infections, necessitating the development of novel therapeutic strategies. The CRISPR/Cas9 gene-editing system has emerged as a revolutionary tool for precision genome modification, offering targeted disruption of antibiotic resistance genes, quorum sensing pathways, and biofilm-regulating factors. However, the clinical application of CRISPR-based antibacterials faces significant challenges, particularly in efficient delivery and stability within bacterial populations. Nanoparticles (NPs) present an innovative solution, serving as effective carriers for CRISPR/Cas9 components while exhibiting intrinsic antibacterial properties. Nanoparticles can enhance CRISPR delivery by improving cellular uptake, increasing target specificity, and ensuring controlled release within biofilm environments. Recent advances have demonstrated that liposomal CRISPR-Cas9 formulations can reduce Pseudomonas aeruginosa biofilm biomass by over 90% in vitro, while gold nanoparticle carriers enhance editing efficiency up to 3.5-fold compared to non-carrier systems. These hybrid platforms also enable co-delivery with antibiotics, producing synergistic antibacterial effects and superior biofilm disruption. Additionally, they can facilitate co-delivery of antibiotics or antimicrobial peptides, further enhancing therapeutic efficacy. This review explores the synergistic integration of CRISPR/Cas9 and nanoparticles in combating biofilm-associated antibiotic resistance. We discuss the mechanisms of action, recent advancements, and current challenges in translating this approach into clinical practice. While CRISPR-nanoparticle hybrid systems hold immense potential for next-generation precision antimicrobial therapies, further research is required to optimize delivery platforms, minimize off-target effects, and assess long-term safety. Understanding and overcoming these challenges will be critical for developing effective biofilm-targeted antibacterial strategies.
Keywords: Antibacterial therapy; Antibiotic resistance; Biofilm; CRISPR/Cas9; Gene editing; Nanoparticles.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
CRISPR/Cas9-targeted smpB mutation revealing roles in biofilm formation, motility, and antibiotic susceptibility in Acinetobacter baumannii.PLoS One. 2025 Aug 4;20(8):e0329638. doi: 10.1371/journal.pone.0329638. eCollection 2025. PLoS One. 2025. PMID: 40758696 Free PMC article.
-
Nanotechnology-Based Delivery of CRISPR/Cas9 for Cancer Treatment: A Comprehensive Review.Cells. 2025 Jul 23;14(15):1136. doi: 10.3390/cells14151136. Cells. 2025. PMID: 40801569 Free PMC article. Review.
-
Respiratory tract antimicrobial peptides more effectively killed multiple methicillin-resistant Staphylococcus aureus and nontypeable Haemophilus influenzae isolates after disruption from biofilm residence.Microbiol Spectr. 2025 Aug 5;13(8):e0306624. doi: 10.1128/spectrum.03066-24. Epub 2025 Jun 18. Microbiol Spectr. 2025. PMID: 40530663 Free PMC article.
-
Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis.Cochrane Database Syst Rev. 2017 Oct 5;10(10):CD009528. doi: 10.1002/14651858.CD009528.pub4. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jun 10;6:CD009528. doi: 10.1002/14651858.CD009528.pub5. PMID: 28981972 Free PMC article. Updated.
References
-
- Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14(9):563–75. - PubMed
-
- Ekwebelem OC, Aleke J, Ofielu E, Nnorom-Dike O. CRISPR-Cas9 system: a revolutionary tool in the fight against antimicrobial resistance: retracted. Infect Microbes Dis. 2021;3(2):51–6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

