SC134-deruxtecan, a fucosyl-GM1 targeting ADC for small cell lung cancer therapy
- PMID: 40830881
- PMCID: PMC12366213
- DOI: 10.1186/s12967-025-06940-2
SC134-deruxtecan, a fucosyl-GM1 targeting ADC for small cell lung cancer therapy
Abstract
Background: Small cell lung cancer (SCLC) remains a difficult disease to treat with poor long-term survival rates. New therapies offer modest overall survival benefit beyond that of chemotherapy alone, necessitating the development of improved therapies. Fucosyl-GM1 (FucGM1) is a glycolipid highly expressed on SCLC cells, but virtually absent in normal tissues, suggesting strong potential for targeted therapy. We have developed SC134-deruxtecan, an antibody drug conjugate (ADC) targeting FucGM1 in SCLC, and characterized its preclinical activity.
Methods: SC134 binding specificity and affinity were tested through enzyme-linked immunosorbent assay (ELISA), surface plasmon resonance (SPR), flow cytometry against several SCLC cell lines, and immunohistochemistry (IHC) of clinical samples and animal tissues. In silico modelling supplemented the FucGM1 binding specificity. Internalization kinetics and colocalization of SC134 with the lysosomes were investigated through imaging flow cytometry. Direct cytotoxicity as well as bystander killing and antibody dependent cell cytotoxicity (ADCC) by SC134-deruxtecan were determined using in vitro cell cytotoxicity assays. SC134-deruxtecan's efficacy was evaluated in vivo using a DMS79 xenograft model.
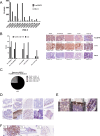
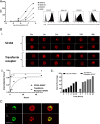
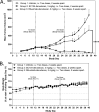
Results: SC134 specifically targets FucGM1, without GM1 cross-reactivity, with nanomolar affinity. In silico modelling of the SC134 FucGM1 binding site revealed a relatively narrow binding pocket, occupied by the terminal three glycans with multiple Fucose-engaging interactions. Robust FucGM1 expression in frozen SCLC patient tissues was evident, whilst tissue cross-reactivity analysis indicated non-human primates as well as mice as suitable tox models. FucGM1 binding by SC134 led to effective internalization, with a 6.9-h half-life, lysosomal colocalization, culminating in sub-nanomolar drug delivery efficiency, across a range of payloads. Covalent deruxtecan conjugation of SC134 with a DAR 8 and a cleavable linker showed effective (nanomolar) in vitro killing of SCLC cell lines such as DMS79 and DMS153, with concentration-dependent bystander killing of FucGM1-negative AGS cells. SCLC cell killing was further augmented through ADCC. Potent in vivo DMS79 xenograft killing was seen at 3mg/kg SC134-deruxtecan, which was well tolerated.
Conclusion: The tumour-specific nature of FucGM1, combined with the potent SCLC killing by SC134-deruxtecan underscore the development potential of SC134 for use as an ADC therapy against SCLC.
Keywords: ADC; Fucosyl-GM1; Glycolipid; Internalization; Small cell lung cancer.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Animal studies complied with the UK Animals Scientific Procedures Act 1986 (ASPA) and were carried out in concordance with Crown Bioscience’s UK Guidelines and Standard Operating Procedures. Patient-derived tumor material was sourced from Crown Bioscience International and was acquired after approval by the IntegReview Ethical Review Board. Patient SCLC tissues were acquired through AccioBiobank, BioMedica CRO and Indivdumed GmbH, according to local institutional review board (IRB) approval and with Informed Consent (IC). Human leukocytes were obtained by BioIVT under IRB approved protocols. All donors signed IC forms. Consent for publication: Not applicable. Competing interests: LD has ownership interest in a patent. LD is a director and shareholder in Scancell Ltd. All other authors are employees of Scancell Ltd.
Figures


References
-
- Basumallik N, Agarwal M. Small cell lung cancer. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2025, StatPearls Publishing LLC. 2025. - PubMed
-
- Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum–etoposide versus platinum–etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51–65. - PubMed
-
- Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929–39. - PubMed
-
- Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(6):739–47. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

