Efficacy evaluation of minimally invasive particle implantation in treating head and neck malignancies under different guidance methods: a propensity score matching analysis study
- PMID: 40830976
- PMCID: PMC12362944
- DOI: 10.1186/s12957-025-03906-y
Efficacy evaluation of minimally invasive particle implantation in treating head and neck malignancies under different guidance methods: a propensity score matching analysis study
Abstract
Purpose: To evaluate the efficacy of minimally invasive particle implantation guided by different methods for head and neck malignancies based on propensity-matched score analysis.
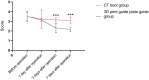
Materials and methods: 120 patients with head and neck malignant tumors treated in our hospital from January 2021 to December 2023 were selected. The patients were matched by Propensity Score Matching (PSM) method at 1:1 and finally included in Computed Tomography (CT) guidance group (n = 60) and 3D print guide group (n = 60).
Results: The pain in the 3D print guide group was significantly lower than that in the CT guidance group at 3 days and 7 days after operation (P < 0.05). Preoperative tumor diameter, postoperative metastasis, and tissue edema were similar. However, the 3D printing guide group had smaller tumor diameters and fewer recurrences (P < 0.05). The 3D printing guide group also showed better curative effects and lower incidence of adverse reactions compared to the CT guidance group (P < 0.05), indicating its superiority in treatment safety and efficacy.
Conclusion: Minimally invasive particle implantation guided by a 3D-printed guide plate is safer and more effective than CT-guided treatment for head and neck malignancies.
Clinical trial number: Not applicable.
Trial registration: Not applicable.
Keywords: Head and neck malignancies; Minimally invasive particle implantation therapy; Propensity score matching; Tropism-matched score.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The patient or family members signed the informed consent form, and this study has been approved by the Ethics Committee of Yichang Second People’s Hospital (Approval No. 202420). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Ahsanuddin S, Jin R, Sheorey L, Sawhney R, Sangal NR, Baredes S, et al. Understanding basal cell adenocarcinoma of the head and neck: Population-based study. Head Neck. 2022;44(2):483–93. - PubMed
-
- Amini A, Verma V, Li R, Vora N, Kang R, Gernon TJ, et al. Factors predicting for patient refusal of head and neck cancer therapy. Head Neck. 2020;42(1):33–42. - PubMed
-
- Farquhar DR, Masood MM, Lenze NR, Tasoulas J, Sheth S, Lumley C, et al. Effect of distance of treatment center on survival for HPV-negative head and neck cancer patients. Head Neck. 2023;45(12):2981–9. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous