Multi-function of adipose-derived stem cells on gut disorder: from bench to bedside
- PMID: 40830985
- PMCID: PMC12366020
- DOI: 10.1186/s13287-025-04549-2
Multi-function of adipose-derived stem cells on gut disorder: from bench to bedside
Abstract
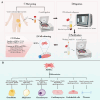
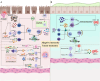
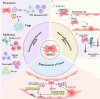
Adipose-derived stem cells (ADSCs) are a specific type of mesenchymal stem cells (MSCs) obtained easily from adipose tissue (AT). Compared with MSCs, ADSCs are easier to obtain, have fewer ethical issues, and have a higher proliferative capacity, which makes them a promising type of stem cell in regenerative medicine. ADSCs possess impressive capabilities in cell regeneration as well as differentiation, making them promising candidates for injury repair, tissue regeneration and alleviation of inflamed tissues. At present, most clinical studies on ADSCs focus on the treatment of wounds, multiple sclerosis, soft tissue trauma, aging, diabetes, Parkinson’s disease, bone and cartilage regeneration, stroke, and spinal cord injury, while its clinical applications in the gastrointestinal tract are relatively few. Therefore, this review summarizes the findings of preclinical experiments, clinical trials, and areas that may require further development of ADSCs in the treatment of digestive disorders, including inflammatory bowel disease (IBD), colorectal cancer (CRC), colorectal fibrosis, hepatocellular carcinoma, hepatic fibrosis, gastric cancer (GC), gastrostomy closure and radiation-induced proctitis. The review is concluded by discussing the goals for improvement and future directions for ADSCs before large-scale clinical application.
Graphical Abstract:
Keywords: Adipose-derived stem cells; Anti-inflammation; Fibrosis; Immunoregulatory effects; Inflammatory bowel disease; Mesenchymal stem cells.
Conflict of interest statement
Declarations. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests. Ethical approval and consent to participate: This systematic review synthesizes existing literature and excludes original research activities, including experiments or data collection involving human subjects or human-derived materials. Ethics approval and consent to participate are not applicable to this manuscript.
Figures



References
-
- Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341–7. - PubMed
-
- Vallée M, Côté JF, Fradette J. Adipose-tissue engineering: taking advantage of the properties of human adipose-derived stem/stromal cells. Pathol Biol (Paris). 2009;57(4):309–17. - PubMed
-
- Shi YY, Nacamuli RP, Salim A, Longaker MT. The osteogenic potential of adipose-derived mesenchymal cells is maintained with aging. Plast Reconstr Surg. 2005;116(6):1686–96. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

