The absence of IL17A favours cytotoxic cell function and improves antigen-specific immunotherapies in pancreatic adenocarcinoma
- PMID: 40831074
- PMCID: PMC12364998
- DOI: 10.1002/ctm2.70442
The absence of IL17A favours cytotoxic cell function and improves antigen-specific immunotherapies in pancreatic adenocarcinoma
Abstract
Background and aims: The pancreatic tumour microenvironment (TME) is a complex ecosystem where tumour cells, cancer-associated fibroblasts and immune cells interact, often in ways that contribute to tumour growth. The role of interleukin (IL17)A in pancreatic cancer progression is now more defined, and it is known to sustain a pro-tumoural microenvironment and inhibit the immune response. Here, we explore the effect of combining IL17A depletion with a cancer vaccine to enhance anti-tumour immunity.
Methods: We used genetically engineered mice proficient or deficient in IL17A, and orthotopically injected mice with pancreatic tumour cells depleted or not in IL17A, to examine the vaccine effects on tumour growth and immune responses. Both humoral and cellular immune responses were analysed following vaccination in IL17A-deficient and control mice.
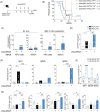
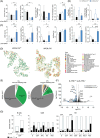
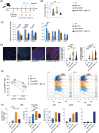
Results: Mice lacking IL17A-either genetically or through pharmacological depletion-exhibited prolonged survival and smaller tumours, compared to vaccinated controls. Vaccination in IL17A-deficient mice significantly increased the influx of immune cells, including Natural Killer (NK) and effector/memory CD8 T cells, which displayed higher cytotoxic activity. CD8 T-cell depletion in these models notably reduced vaccine efficacy, underscoring the essential role of these cells. NK cell depletion in untreated models further demonstrated NK cells' critical function in controlling tumour growth when IL17A was absent. Overall, IL17A depletion enhanced both antigen-specific humoral and cellular immune responses, indicating a shift towards a more robust and responsive immune environment.
Conclusions: Our findings reveal that the absence of IL17A in the pancreatic TME reprograms it into a more immune-supportive environment, favouring the recruitment of effector/memory immune cells upon vaccination. This approach paves the way for novel therapeutic combinations in pancreatic cancer, where IL17A depletion may boost both immunotherapy efficacy and anti-tumour responses.
Keywords: IL17A; cancer vaccine; humoral response; pancreatic cancer; tumour immune microenvironment.
© 2025 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
