Safety and effectiveness of the early-onset sepsis calculator to reduce antibiotic exposure in at-risk newborns: a cluster-randomised controlled trial
- PMID: 40831464
- PMCID: PMC12359153
- DOI: 10.1016/j.eclinm.2025.103419
Safety and effectiveness of the early-onset sepsis calculator to reduce antibiotic exposure in at-risk newborns: a cluster-randomised controlled trial
Abstract
Background: Newborns are at risk for early-onset sepsis (EOS), occurring 0.2-2.0 per 1000 live births, and for antibiotic overtreatment: approximately 5-15% receive antibiotics for suspected EOS under conventional guidelines with categorical risk factor assessment. Use of the multivariate neonatal EOS calculator prediction tool can reduce overtreatment, but no trials have been conducted to compare its safety to these categorical guidelines.
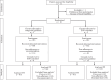
Methods: Between April 12th, 2022, and March 19th, 2024, we conducted an open-label, two-armed, cluster-randomised controlled trial among newborns born at ≥34 weeks' gestational age with ≥1 EOS risk factor, comparing 10 hospitals randomised 1:1 to EOS calculator use versus categorical guideline use (ClinicalTrials.gov number: NCT05274776). The EOS calculator was slightly adapted for Dutch use. The co-primary non-inferiority outcome assessed safety using four predefined harm criteria (respiratory support, circulatory support, referral to intensive care unit, and culture-confirmed EOS). Non-inferiority was established if the upper limit of the 95% confidence interval (CI) for the relative risk did not exceed 1.5. The co-primary superiority outcome assessed the reduction of participants starting antibiotic therapy for suspected EOS within 24 h postpartum. Secondary endpoints were the duration of antibiotic therapy and the initiation of antibiotic therapy between 24 and 72 h after birth. Intention-to-treat and per-protocol analyses were performed.
Findings: 1830 newborns (183 per cluster) were included. At least one harm criterion was present in 64 (7.0%) of 915 in the EOS calculator arm and 134 (14.6%) of 915 in the categorical guideline arm (relative risk 0.48; 95% Cl 0.36-0.63). Antibiotics for suspected EOS were started in 66 (7.2%) of 915 in the EOS calculator arm, compared with 243 (26.6%) of 915 in the categorical guideline arm (absolute risk reduction: 19.0%, 95% CI 11.3-26.7). Median duration of antibiotics was longer in the EOS calculator arm (5.5 days, IQR 1.8-6.6) than in the categorical guideline arm (2.1 days, IQR 1.6-6.3) (P 0.0019). We found no difference in the proportion of newborns started on antibiotic therapy for suspected EOS between 24 and 72 h after birth. Adverse event rates were similar between arms. Readmission for suspected early-onset sepsis occurred three times in the EOS calculator and two times in the categorical guideline arm. Any cultures obtained at readmission remained negative, and any symptoms resolved completely.
Interpretation: These trial data support safety and effectiveness of the EOS calculator for harm criteria and for the proportion of participants that started antibiotic therapy.
Funding: This study was supported by SPIN, the General Paediatrics Research Network of the Dutch Association for Paediatrics, supported by het Cultuurfonds.
Keywords: EOS; Early-onset sepsis; Early-onset sepsis calculator; Neonatal sepsis calculator.
© 2025 The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures
References
-
- Strunk T., Molloy E.J., Mishra A., Bhutta Z.A. Neonatal bacterial sepsis. Lancet. 2024;404(10449):277–293. - PubMed
-
- National Institute for Health and Care Excellence (NICE) National Institute for Health and Care Excellence (NICE); London, UK: 2012. Neonatal infection (early onset): antibiotics for prevention and treatment (CG149)
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous