This is a preprint.
Novel Papain-Elastase Induced Murine Model for Infrarenal Abdominal Aortic Aneurysm Rupture
- PMID: 40831493
- PMCID: PMC12363923
- DOI: 10.21203/rs.3.rs-7166564/v1
Novel Papain-Elastase Induced Murine Model for Infrarenal Abdominal Aortic Aneurysm Rupture
Abstract
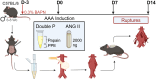
Abdominal aortic aneurysm (AAA) rupture leads to high morbidity and mortality. Current rodent models struggle to reliably mimic infrarenal AAA rupture. Chemical treatments using porcine pancreatic elastase (PPE), papain (Pap), β-aminopropionitrile (BAPN), and angiotensin II (ANG II) are known to induce AAA in rodents. We hypothesized that combining these agents could establish reliable chronic AAA and acute rupture models, resembling human pathology. Here AAAs were induced in male C57BL/6 mice using peri-adventitial exposure to PPE, Pap, or a combination (PPE + Pap), with or without 0.3% BAPN and ANG II. Two weeks post-induction, Pap and PPE + Pap showed increased aortic diameters, higher inflammation, elastase degradation, and matrix metallopeptidase (MMP) activity. Addition of BAPN resulted in large chronic AAAs (500% growth) and intraluminal thrombus (ILT) formation. ANG II-treated mice exhibited a 93% rupture rate, increased inflammation, MMP activation, and ILT formation. These novel murine models are ideal for investigating AAA pathophysiology and therapeutic discovery.
Conflict of interest statement
Conflict of Interest: The authors declare no relevant conflicts of interest.
Figures





Similar articles
-
Advanced Abdominal Aortic Aneurysm Modeling in Mice by Combination of Topical Elastase and Oral ß-aminopropionitrile.J Vis Exp. 2024 Jul 26;(209):10.3791/66812. doi: 10.3791/66812. J Vis Exp. 2024. PMID: 39141527 Free PMC article.
-
Small AAAs: Recommendations for Rodent Model Research for the Identification of Novel Therapeutics.Arterioscler Thromb Vasc Biol. 2024 Jul;44(7):1467-1473. doi: 10.1161/ATVBAHA.124.320823. Epub 2024 Jun 26. Arterioscler Thromb Vasc Biol. 2024. PMID: 38924435 Free PMC article. Review.
-
A novel chronic advanced stage abdominal aortic aneurysm murine model.J Vasc Surg. 2017 Jul;66(1):232-242.e4. doi: 10.1016/j.jvs.2016.07.105. Epub 2017 Mar 6. J Vasc Surg. 2017. PMID: 28274752 Free PMC article.
-
Establishment of a New Abdominal Aortic Aneurysm Model in Rats by a Retroperitoneal Approach.Front Cardiovasc Med. 2022 Feb 23;9:808732. doi: 10.3389/fcvm.2022.808732. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35282381 Free PMC article.
-
A systematic review summarizing local vascular characteristics of aneurysm wall to predict for progression and rupture risk of abdominal aortic aneurysms.J Vasc Surg. 2023 Jan;77(1):288-298.e2. doi: 10.1016/j.jvs.2022.07.008. Epub 2022 Jul 14. J Vasc Surg. 2023. PMID: 35843510
References
-
- Kent K. C. et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 52, 539–548 (2010). - PubMed
-
- Moll F. L. et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. European Journal of Vascular and Endovascular Surgery 41, (2011). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

