Butein Alleviates Non-Alcoholic Steatohepatitis in Leptin-Deficient Mice by Modulating the PDE4/cAMP/p-CREB Pathway
- PMID: 40831700
- PMCID: PMC12360368
- DOI: 10.2147/DDDT.S530855
Butein Alleviates Non-Alcoholic Steatohepatitis in Leptin-Deficient Mice by Modulating the PDE4/cAMP/p-CREB Pathway
Abstract
Purpose: Non-alcoholic steatohepatitis (NASH) is a prevalent liver disease characterized by steatosis, inflammation, and liver injury. Despite its increasing incidence, effective treatments are limited. Butein, a flavonoid with anti-cancer, anti-inflammatory, and antioxidant properties, has not been thoroughly studied for its potential therapeutic effects in NASH. This study aimed to evaluate the effects of butein in NASH using both in vivo and in vitro experimental models, with emphasis on elucidating the underlying molecular signaling mechanisms.
Methods: The leptin-deficient (ob/ob) mouse model of NASH, induced by the Gubra amylase NASH (GAN) diet, was employed to assess the therapeutic effects and mechanistic pathways of butein treatment. In vitro investigations utilized palmitic acid-induced HepG2 human hepatocellular carcinoma cells and LX-2 hepatic stellate cells to explore butein's impact on oxidative stress, inflammatory responses, and fibrotic processes.
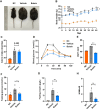
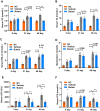
Results: Butein treatment resulted in significant amelioration of glucolipid metabolism dysregulation, hepatic inflammation, and liver fibrosis in the mouse model, potentially mediated through modulation of the PDE4/cAMP/p-CREB signaling pathway. In in vitro experimental models, butein effectively attenuated lipid-induced oxidative stress in HepG2 cells and reduced inflammatory and fibrotic responses in LX-2 cells, demonstrating consistent protective effects across both experimental models.
Conclusion: These findings establish the protective effects of butein against NASH progression through PDE4/cAMP/p-CREB pathway modulation, supporting its potential as a therapeutic candidate for NASH treatment pending further clinical validation.
Keywords: fibrosis; inflammation; lipid metabolism; non-alcoholic fatty liver disease.
© 2025 Guo et al.
Conflict of interest statement
The authors declare that no potential conflict of interest that could have appeared to influence the work reported in this paper.
Figures







References
-
- Smith A, Baumgartner K, Bositis C. Cirrhosis: diagnosis and management. Am Fam Physician. 2019;100(12):759–770. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

