Mating status-dependent dopaminergic modulation of auditory sensory neurons in Drosophila
- PMID: 40831745
- PMCID: PMC12358670
- DOI: 10.1016/j.isci.2025.113232
Mating status-dependent dopaminergic modulation of auditory sensory neurons in Drosophila
Abstract
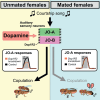
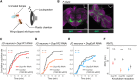
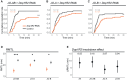
Mating status often modulates responses to courtship sounds in animals. The neural mechanisms underlying this modulation, however, have not been well clarified. Here, we show that dopaminergic signals are involved in modulating the responses of auditory sensory neurons in Drosophila melanogaster females depending on their mating status. These neurons abundantly express three types of dopamine receptors, with some having direct synaptic connections with dopaminergic neurons. Of these receptors, suppressing the expression of Dop1R2 reduces sound responses of auditory sensory neurons in unmated but not mated females. Moreover, the expression of Dop1R2 in auditory sensory neurons enhances the song response behavior of unmated females, manifested by copulation receptivity when exposed to songs. Our research suggests that dopaminergic modulation via Dop1R2 is involved in mating the state-dependent regulation of auditory sensory processing.
Keywords: Behavioral neuroscience; Biological sciences; Natural sciences; Neuroscience; Sensory neuroscience.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

