RNA signaling in skeletal muscle: the central role of microRNAs and exosomal microRNAs
- PMID: 40831752
- PMCID: PMC12358497
- DOI: 10.3389/fcell.2025.1639123
RNA signaling in skeletal muscle: the central role of microRNAs and exosomal microRNAs
Abstract
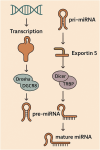
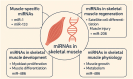
Skeletal muscle development and adaptation are governed by complex regulatory networks that coordinate gene expression, signaling pathways, and intercellular communication. Among the emerging key regulators are microRNAs (miRNAs) and exosomal microRNAs, which function as critical modulators of skeletal muscle growth, differentiation, regeneration, and metabolic adaptation. The review explores the acknowledged contributions of miRNAs, both intracellular and those encapsulated within exosomes, to the regulation of skeletal muscle physiology. We highlight their involvement in major molecular pathways, including PI3K/Akt/mTOR, TGF-β/Smad, Wnt/β-catenin, and AMPK signaling, and their impact on processes such as myogenesis, hypertrophy, atrophy, and mitochondrial function. Emphasis is placed on the critical role of exosomal miRNAs in orchestrating signaling pathways that enable communication among cells in the muscle milieu and with peripheral tissues. Ultimately, the review addresses the clinical relevance of miRNAs, including those derived from exosomes, emphasizing their prospective roles as diagnostic tools and intervention points in muscle-related conditions. In sum, the review elucidates the broad landscape of RNA-related regulatory processes in skeletal muscle and projects forward-looking strategies for translational exploration in this rapidly developing scientific domain.
Keywords: aging; epigentic; exosome; microRNA; skeletal muscle.
Copyright © 2025 Liu and Dong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Amirouche A., Jahnke V. E., Lunde J. A., Koulmann N., Freyssenet D. G., Jasmin B. J. (2017). Muscle-specific microRNA-206 targets multiple components in dystrophic skeletal muscle representing beneficial adaptations. Am. J. Physiology-Cell Physiology 312 (3), C209–C21. 10.1152/ajpcell.00185.2016 - DOI - PubMed
-
- Amirouche A., Tadesse H., Miura P., Belanger G., Lunde J. A., Côté J., et al. (2014). Converging pathways involving microRNA-206 and the RNA-binding protein KSRP control post-transcriptionally utrophin A expression in skeletal muscle. Nucleic acids Res. 42 (6), 3982–3997. 10.1093/nar/gkt1350 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

