Immunometabolic Targets in CD8+ T Cells within the Tumor Microenvironment of Hepatocellular Carcinoma
- PMID: 40831886
- PMCID: PMC12360748
- DOI: 10.1159/000542578
Immunometabolic Targets in CD8+ T Cells within the Tumor Microenvironment of Hepatocellular Carcinoma
Abstract
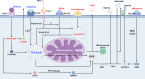
Background: CD8+ T cells are critical for the oncogenesis and progression of the hepatocellular carcinoma (HCC) tumor microenvironment, receiving antigen signals from antigen-presenting cells and directly contributing to antitumor responses.
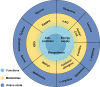
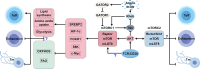
Summary: CD8+ T cells mediate immunogenic cell death, facilitate immune signal transmission, and play a significant role in various treatments, including surgery, transarterial chemoembolization, and immunotherapy. Extensive research on the role of CD8+ T cells within the HCC microenvironment has shown considerable progress. Immunometabolic targets on CD8+ T cells have demonstrated potential in combination with immunotherapies for HCC; however, they have not yet reached the clinical trial stage.
Key messages: This review provides a comprehensive overview of recent research on immune and immunometabolic targets of CD8+ T cells within the HCC microenvironment. By highlighting advances and potential mechanisms, this review aims to support the development of effective clinical strategies in this field.
Keywords: CD8+ T cell; Hepatocellular carcinoma; Immunotherapy; Mammalian target of rapamycin; Metabolism; Tumor microenvironment.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. - PubMed
-
- Ladd AD, Duarte S, Sahin I, Zarrinpar A. Mechanisms of drug resistance in HCC. Hepatology. 2024;79(4):926–40. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. - PubMed
-
- Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. 2022;21(7):509–28. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

