Systematic literature review of real-world evidence on overall survival in cancer patients before and after the approval of anti-PD-(L)1 therapy
- PMID: 40831930
- PMCID: PMC12358273
- DOI: 10.3389/fonc.2025.1615795
Systematic literature review of real-world evidence on overall survival in cancer patients before and after the approval of anti-PD-(L)1 therapy
Abstract
Background: The development and regulatory approval of anti-programmed death (ligand) 1 (anti-PD-(L)1) agents, based on positive clinical trial results, has dramatically changed clinical practice and treatment paths in oncology. However, the effectiveness of anti-PD-(L)1 therapy in real-world settings is not well understood. Therefore, it is important to summarize real-world evidence on the overall survival (OS) of patients with specific tumor types prior to and following the regulatory approval of anti-PD-(L)1 therapy.
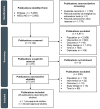
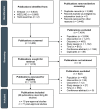
Methods: A systematic literature review including observational studies worldwide reporting the OS of patients receiving conventional first-line pharmacological therapy for advanced/metastatic non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), or melanoma in the anti-PD-(L)1 pre-approval era and similar patients receiving first-line anti-PD-(L)1 therapy in the post-approval era was conducted. For each tumor type, studies were selected from a pre-approval era, defined as a period beginning 5 years before the first approval of an anti-PD-(L)1 agent and ending the year before its approval for first-line therapy, and a post-approval era, defined as a period beginning the year that an anti-PD-(L)1 agent was approved for first-line therapy and ending in 2023. Relevant studies were identified through MEDLINE and Embase searches. Study selection, data extraction, and quality assessment were conducted by two independent reviewers. Median OS (mOS) was summarized within each tumor type and descriptively compared across the pre- and post-approval eras.
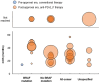
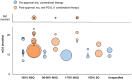
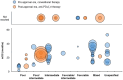
Results: A total of 86, 44, and 35 studies evaluating first-line treatments for advanced/metastatic NSCLC, RCC, and melanoma, respectively, were included. Post-approval mOS in patients treated with anti-PD-(L)1 therapy tended to be numerically longer than pre-approval mOS in patients treated with conventional therapy within certain patient and treatment categories. For example, pre-approval mOS ranged from 6.9 to 18.4 months (n=18 treatment groups), and post-approval mOS ranged from 10.6 to 46.2 months in NSCLC patients with PD-L1 tumor expression ≥50% who received anti-PD-(L)1 monotherapy (n=33; with mOS not reached for n=3). In RCC patients classified as high-risk, pre-approval mOS ranged from 2 to 10.3 months (n=7), and post-approval mOS ranged from 7.8 to 24.3 months (n=4). Also, in melanoma patients with any BRAF mutation, pre-approval mOS was 14.2 months (n=1), and post-approval mOS ranged from 15.9 to 51.2 months (n=6; with mOS not reached for n=3).
Conclusion: A survival benefit in real-world practice was observed for patients with advanced/metastatic NSCLC, RCC, or melanoma receiving first-line anti-PD-(L)1 therapy after its regulatory approval when compared with patients treated with conventional care before anti-PD-(L)1 therapy approval. This supports the use of anti-PD-(L)1 therapy as a standard of care in many countries.
Keywords: anti-PD-1; anti-PD-L1; immune checkpoint inhibitors; melanoma; non-small cell lung cancer; overall survival; real-world evidence; renal cell carcinoma.
Copyright © 2025 Nayak, Akers, Frederickson, Mbous and Aguiar-Ibáñez.
Conflict of interest statement
RA-I is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and a shareholder of Merck & Co., Inc., Rahway, NJ, USA. YM was an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, at the time the study was conducted. DN, KA, and AF are employees of Precision AQ, a healthcare research consulting firm that received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, to conduct the research described in this manuscript.
Figures

References
-
- Tan YY, Papez V, Chang WH, Mueller SH, Denaxas S, Lai AG. Comparing clinical trial population representativeness to real-world populations: an external validity analysis encompassing 43 895 trials and 5 685 738 individuals across 989 unique drugs and 286 conditions in England. Lancet Healthy Longevity. (2022) 3:e674–89. doi: 10.1016/S2666-7568(22)00186-6, PMID: - DOI - PubMed
-
- Iacovino ML, Celant S, Tomassini L, Arenare L, Caglio A, Canciello A, et al. Comparison of baseline patient characteristics in Italian oncology drug monitoring registries and clinical trials: a&xa0;real-world cross-sectional study. Lancet Regional Health – Europe. (2024) 41:100912. doi: 10.1016/j.lanepe.2024.100912, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

