This is a preprint.
VO: The Vaccine Ontology
- PMID: 40832256
- PMCID: PMC12363946
- DOI: 10.1101/2025.08.12.669998
VO: The Vaccine Ontology
Abstract
With the widespread use of vaccines in research and clinical settings, there is an urgent need to standardize vaccine representation, integrate information across diverse vaccine types, and support computer-assisted reasoning. Accordingly, we have since 2007 developed the community-based Vaccine Ontology (VO), which aligns with the Basic Formal Ontology and adheres to OBO Foundry principles. VO models ontologically vaccines, vaccine components, vaccine immune responses, vaccine investigation studies and other vaccine-related topics. VO represents more than 10,000 vaccines targeting 289 infectious pathogens and cancers in humans and over 30 nonhuman animal species. VO provides mappings to external resources such as RxNorm, CVX, FDA, and USDA. Various VO use cases exist. VO facilitates vaccine standardization in resources such as the VIOLIN vaccine database, ImmPort, and the Vaccine Adjuvant Compendium (VAC). Semantic queries can be made to query VO. VO has been shown to enhance experimental and clinical vaccine data analysis and vaccine literature mining. Overall, VO standardizes vaccine modeling and representation and greatly supports vaccine AI research in the Semantic Web era.
Keywords: FAIR; Semantic Web; VO; Vaccine Ontology; ontology; vaccination; vaccine.
Conflict of interest statement
Competing Interests Dr. Yongqun He (Oliver) is an Editorial Board Member for Scientific Data, and has served as a Guest Editor for the “Data standards and ontologies for clinical and medical research” Collection.
Figures
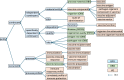
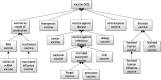

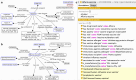
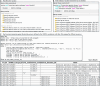

References
-
- Centers for Disease Control and Prevention. CVX (Vaccine Administered), https://www2.cdc.gov/vaccines/iis/iisstandards/vaccines.asp?rpt=cvx (2024).
-
- U.S. Food and Drug Administration. FDA Approved Vaccines, https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-... (2025).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
