This is a preprint.
The TECTB-C225Y Variant Causing Autosomal Dominant Deafness in a Nicaraguan Family Enhances Sensitivity to Noise-Induced Hearing Loss in Mice
- PMID: 40832383
- PMCID: PMC12363751
- DOI: 10.1101/2025.08.13.25333146
The TECTB-C225Y Variant Causing Autosomal Dominant Deafness in a Nicaraguan Family Enhances Sensitivity to Noise-Induced Hearing Loss in Mice
Abstract
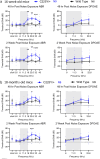
Identifying new genes responsible for non-syndromic hearing loss remains a critical goal, as many individuals with hereditary deafness still lack a molecular diagnosis despite comprehensive genetic testing. The tectorial membrane (TM) is a specialized, collagen-rich, acellular matrix of the inner ear, essential for stimulating mechanosensitive hair cell bundles during sound transduction, and its structural integrity is critical for frequency tuning and auditory sensitivity. Although mutations in genes encoding a number of non-collagenous proteins found in the TM (TECTA, CEACAM16, OTOG, OTOGL) have been identified as deafness genes, definitive evidence implicating β-tectorin (TECTB) in human hearing loss has been lacking. Here, we present multiple lines of genetic and experimental evidence linking a missense variant in TECTB (c.674G>A, p.Cys225Tyr) to autosomal dominant, non-syndromic hearing loss in a multigenerational family. The variant alters one of eight highly conserved cysteines present within the zona pellucida (ZP) domain of TECTB and is predicted to disrupt protein folding and matrix assembly. Using a Tectb-C225Y knock-in mouse model, we show that homozygous animals exhibit severe hearing loss and profound disruption of TM morphology, while heterozygote animals display decreased matrix content within the TM and increased susceptibility to noise-induced hearing loss-despite normal auditory thresholds. These findings identify TECTB as a novel human deafness gene, further elucidate its structural role in maintaining TM integrity, and highlight its contribution to resilience against environmental and age-related auditory decline.
Keywords: Hearing loss; beta-tectorin (TECTB); deafness; striated sheet matrix; tectorial membrane.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures





References
-
- Armstrong N. M., Wang H., E J. Y., Lin F. R., Abraham A. G., Ramulu P., Resnick S. M., Tian Q., Simonsick E., Gross A. L., Schrack J. A., Ferrucci L., & Agrawal Y. (2022). Patterns of Prevalence of Multiple Sensory Impairments Among Community-dwelling Older Adults. J Gerontol A Biol Sci Med Sci, 77(10), 2123–2132. 10.1093/gerona/glab294 - DOI - PMC - PubMed
-
- Azaiez H., Decker A. R., Booth K. T., Simpson A. C., Shearer A. E., Huygen P. L., Bu F., Hildebrand M. S., Ranum P. T., Shibata S. B., Turner A., Zhang Y., Kimberling W. J., Cornell R. A., & Smith R. J. (2015). HOMER2, a stereociliary scaffolding protein, is essential for normal hearing in humans and mice. PLoS Genet, 11(3), e1005137. 10.1371/journal.pgen.1005137 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
