Design and Enzyme-Targeted Assessment of 1,2,4-Triazole-1,8-Naphthalimide Hybrids in Drug Discovery
- PMID: 40832451
- PMCID: PMC12358230
- DOI: 10.1155/bmri/6115993
Design and Enzyme-Targeted Assessment of 1,2,4-Triazole-1,8-Naphthalimide Hybrids in Drug Discovery
Abstract
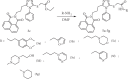
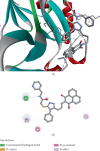
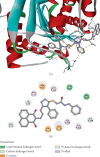
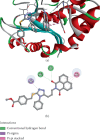
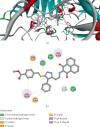
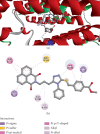
This study reports the design, synthesis, and biological evaluation of novel hybrid 1,2,4-triazole-1,8-naphthalimide derivatives using both classical and environmentally friendly synthetic routes. The synthetic strategy involved a multistep process starting with the preparation of triazolylthioacetic acid esters, followed by electrophilic cyclization-employing both conventional bromine and a green, bromine-free method-and culminating in amidation reactions to yield the target compounds. Structural modifications, including the incorporation of pyridinyl and benzoate moieties, were introduced to enhance biological activity. The compounds were evaluated for antimicrobial and anti-inflammatory activities. In antimicrobial assays, several derivatives demonstrated selective activity, with Compound 5f showing the strongest broad-spectrum antibacterial effects, particularly against Bacillus cereus and Lactobacillus plantarum, and Compound 3e displaying notable dual-action activity against both bacterial and fungal strains. These observations underscore the influence of specific functional groups on microbial targeting and membrane penetration. Anti-inflammatory potential was assessed via IL-6 inhibition using ELISA. Significant reductions in IL-6 levels were observed for Compounds 3a, 3c, 3e, 4, 5c, 5f, and 5g, indicating promising activity, with Compounds 5c and 3a reducing IL-6 levels to 7 pg/mL. The complementary molecular docking studies revealed strong binding affinities for Compound 5f across multiple bacterial enzymes, suggesting effective interactions through hydrogen bonding, electrostatic, and hydrophobic forces and supporting its potential to target iron-regulated pathways and fosfomycin resistance mechanisms. Overall, the integrative approach combining synthetic chemistry, biological assays, and computational modeling highlights the potential of these hybrid 1,2,4-triazole derivatives as candidates for further development as antimicrobial and anti-inflammatory agents.
Copyright © 2025 Nataliya Korol et al. BioMed Research International published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Sathyanarayana R., Poojary B. Exploring Recent Developments on 1,2,4-Triazole: Synthesis and Biological Applications. Journal of the Chinese Chemical Society . 2020;67(4):459–477. doi: 10.1002/jccs.201900304. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

