Spastic Ataxia Composite (SPAXCOM): A Scale to Evaluate the Progression of Subjects with Spasticity and Ataxia
- PMID: 40832806
- PMCID: PMC12661626
- DOI: 10.1002/mds.70006
Spastic Ataxia Composite (SPAXCOM): A Scale to Evaluate the Progression of Subjects with Spasticity and Ataxia
Abstract
Background: Current clinical scales that track disease progression are more tailored to spasticity or ataxia, with limited sensitivity to change.
Objectives: The aim was to develop a sensitive and valid scale specifically geared towards optimized sensitivity to change and adapted to patients presenting with both spasticity and ataxia.
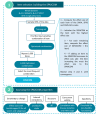
Methods: Longitudinal data from 127 spastic paraplegia type 7 (SPG7) and 112 autosomal recessive spastic ataxia Charlevoix-Saguenay (ARSACS) patients were collected within the multicenter PROSPAX study. Sensitivity to change over 2 years of 30 items from the Scale for the Rating and Assessment of Ataxias (SARA), Spastic Paraplegia Rating Scale (SPRS), and the Activities of Daily Living subscale of the Friedreich's Ataxia Rating Scale (FARS-ADL) was evaluated. Items that demonstrated the highest sensitivity to change were used to build the Spastic Ataxia Composite scale (SPAXCOM).
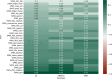
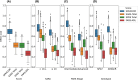
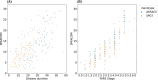
Results: With seven items, the SPAXCOM showed an effect size of 0.71, higher than reference scales (SARA: 0.43, SPRS: 0.42, FARS-ADL: 0.27). The SPAXCOM had a similar sensitivity to change for both genotypes and was more sensitive in participants with a SARA lower than 10 and within the intermediate disease stage (FARS-Disease Staging: 2-3.5). The SPAXCOM showed a high correlation with disease duration (r = 0.67 [0.60; 0.72]) and disease stage (r = 0.86 [0.83; 0.89]). Perception of improvement, stagnation, and worsening were associated with a mean yearly SPAXCOM change of 0.44 (-0.14; 1.01), 0.61 (0.19; 1.03), and 1.22 (0.96; 1.49), respectively.
Conclusion: The SPAXCOM is more sensitive to change and homogeneous across genotypes than the reference scales, allowing a reduction of the required sample size in future clinical trials. © 2025 The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Keywords: SPG7; ataxia; disease progression; spastic ataxia; spastic ataxia Charlevoix‐Saguenay type.
© 2025 The Author(s). Movement Disorders published by Wiley Periodicals LLC on behalf of International Parkinson and Movement Disorder Society.
Figures
References
-
- Pedroso JL, Vale TC, França Junior MC, Kauffman MA, Teive H, Barsottini OGP, Munhoz RP. A diagnostic approach to spastic ataxia syndromes. Cerebellum 2022;21(6):1073–1084. - PubMed
-
- Traschütz A, Adarmes‐Gomez AD, Anheim M, et al. Autosomal recessive cerebellar ataxias in Europe: frequency, onset, and severity in 677 patients. Mov Disord 2023;38(6):1109–1112. - PubMed
-
- Perez‐Lloret S, Warrenburg B, Rossi M, et al. Assessment of ataxia rating scales and cerebellar functional tests: critique and recommendations. Mov Disord 2021;36(2):283–297. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

