Baseline characteristics of atopic eczema patients enrolled in seven European registries united in the TREatment of ATopic eczema (TREAT) registry taskforce
- PMID: 40832875
- PMCID: PMC12645184
- DOI: 10.1111/jdv.20876
Baseline characteristics of atopic eczema patients enrolled in seven European registries united in the TREatment of ATopic eczema (TREAT) registry taskforce
Abstract
Background: The TREAT Registry Taskforce is a collaborative effort of international registries aiming to provide real-world data on the long-term efficacy, cost-effectiveness and safety of systemic treatments and phototherapy for atopic eczema (AE).
Objectives: This study seeks to present a comprehensive overview of the demographics, prior systemic treatments, clinical characteristics and disease severity and burden at baseline among patients enrolled in seven TREAT registries. Moreover, the aim is to gain insight into the differences between the registries and to explore the current prescribing practices of various therapies for patients with AE across Europe.
Methods: Data from June 2016 to 31 October 2022, were collected from seven observational cohorts: A-STAR (UK/Ireland), AtopyReg (Italy), Biobadatop (Spain), SCRATCH (Denmark), SwedAD (Sweden), TREATgermany (Germany) and TREAT NL/BE (Netherlands/Belgium).
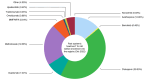
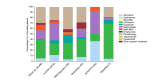
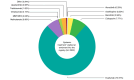
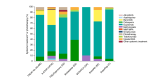
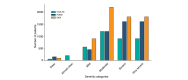
Results: The analysis included 5337 patients, with a mean age of 39.1 years (6.3% paediatric, 54.4% male). Of these, 84.1% had previously received systemic treatments, primarily systemic corticosteroids (58.8%) and ciclosporin (39.0%), while 30.1% had undergone phototherapy. At enrolment, dupilumab was the most prescribed treatment (75.0%), followed by ciclosporin (7.8%) and Janus Kinase inhibitors (5.9%); only 1.7% started phototherapy. Baseline assessments showed that most patients had moderate (41.9%) to severe (30.1%) AE, with an average Eczema Area and Severity Index (EASI) score of 17.6. The Patient-Oriented Eczema Measure (POEM) score averaged 17.2, indicating severe disease impact. The Dermatology Life Quality Index (DLQI) score averaged 13.4, and the Numerical Rating Scale (NRS) for itch was 6.4.
Conclusions: This pooled analysis from the TREAT Registry Taskforce highlights the variability and similarities in data collection across national registries, providing significant insights into the baseline characteristics of the patient population. It establishes a robust foundation for future analyses of key effectiveness and safety outcomes.
Keywords: atopic dermatitis; atopic eczema; baseline; demographics; disease burden; disease severity; patient‐reported outcomes; phototherapy; real‐world data; registry; systemic immunomodulating treatment.
© 2025 The Author(s). Journal of the European Academy of Dermatology and Venereology published by John Wiley & Sons Ltd on behalf of European Academy of Dermatology and Venereology.
Conflict of interest statement
L. A. A. Gerbens: one of the main investigators of the TREAT NL/BE registry. She has no further conflicts of interest. L. F. van der Gang: is a speaker for Abbvie and Sanofi. M. A. Middelkamp‐Hup: consultancies for Sanofi and Leo Pharma and one of the main investigators of the TREAT NL/BE registry. D. J. Hijnenis or has been a consultant and/or investigator for Abbvie, Almirall, Astrazeneca, Galderma, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sanofi, UCB. C. Flohris Chief Investigator of the UK National Institute for Health Research–funded TREAT (ISRCTN15837754) and SOFTER (
Figures
References
-
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the nomenclature review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113(5):832–836. - PubMed
-
- Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC phase three. J Allergy Clin Immunol. 2009;124(6):1251–1258.e23. - PubMed
-
- Langan SM, Alan ID, Weidinger S. Atopic dermatitis. Lancet. 2020;369:345–360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

