The effects of switching from etelcalcetide to upacicalcet in hemodialysis patients with secondary hyperparathyroidism
- PMID: 40833047
- PMCID: PMC12573280
- DOI: 10.5414/CN111695
The effects of switching from etelcalcetide to upacicalcet in hemodialysis patients with secondary hyperparathyroidism
Abstract
Aims: No English-language research papers have reported on the clinical use of upacicalcet, a novel intravenous calcimimetic agent for the treatment of secondary hyperparathyroidism (SHPT) in hemodialysis patients. Therefore, this study aimed to investigate the outcomes of switching from etelcalcetide to upacicalcet.
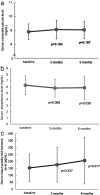
Materials and methods: The subjects included 37 hemodialysis patients with SHPT treated with etelcalcetide before switching to upacicalcet. This study was a single-center retrospective study conducted in Japan. Serum levels of corrected calcium (Ca), phosphorus (P), intact parathyroid hormone (iPTH), and the dose of maxacalcitol were assessed at 3 and 6 months after switching to upacicalcet.
Results: As a result of the switch from etelcalcetide to upacicalcet, the serum corrected Ca level remained unchanged, from 8.9 ± 0.6 to 9.1 ± 0.7 mg/dL (p = 0.104) at 3 months and to 9.0 ± 0.6 mg/dL (p = 0.197) at 6 months. Meanwhile, the serum P level decreased from 6.3 ± 1.5 to 5.8 ± 1.5 mg/dL (p = 0.069) at 3 months and to 5.9 ± 1.9 mg/dL (p = 0.039) at 6 months. The iPTH level increased slightly, from 153.8 ± 100.3 pg/mL to 176.4 ± 124.6 pg/mL (p = 0.337) at 3 months and to 206.5 ± 168.7 pg/mL (p = 0.017) at 6 months. Multiple regression analysis revealed that the change in iPTH was related to the change in P levels.
Conclusion: These findings suggested that upacicalcet may be a useful option for managing serum P levels in hemodialysis patients with SHPT.
Conflict of interest statement
This study was conducted with funds provided by Sanwa Kagaku Kenkyusho Co., Ltd., according to the contract; however, this did not have an impact on the conduct or evaluation of this study. In addition, funds were not provided to the study facility or the investigators. Sanwa Kagaku Kenkyusho Co., Ltd., only provided funds for the outsourcing of the operations.
Figures


References
-
- Moe SM Drüeke TB Management of secondary hyperparathyroidism: the importance and the challenge of controlling parathyroid hormone levels without elevating calcium, phosphorus, and calcium-phosphorus product. Am J Nephrol. 2003; 23: 369–379. - PubMed
-
- Cunningham J Locatelli F Rodriguez M Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011; 6: 913–921. - PubMed
-
- Akimbekov NS Digel I Sherelkhan DK Razzaque MS Vitamin D and Phosphate Interactions in Health and Disease. Adv Exp Med Biol. 2022; 1362: 37–46. - PubMed
-
- Frazão JM Martins P Coburn JW The calcimimetic agents: perspectives for treatment. Kidney Int Suppl. 2002; 61: 149–154. - PubMed
-
- Harada K Fujioka A Konno M Inoue A Yamada H Hirota Y Pharmacology of Parsabiv® (etelcalcetide, ONO-5163/AMG 416), a novel allosteric modulator of the calcium-sensing receptor, for secondary hyperparathyroidism in hemodialysis patients. Eur J Pharmacol. 2019; 842: 139–145. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

