Type-specific EV-D68 real-time RT-PCR assay for the detection of all extant enterovirus D68 strains
- PMID: 40833090
- PMCID: PMC12421836
- DOI: 10.1128/jcm.01492-22
Type-specific EV-D68 real-time RT-PCR assay for the detection of all extant enterovirus D68 strains
Abstract
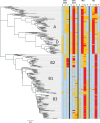
We developed a type-specific Enterovirus D68 (EV-D68) real-time RT-PCR (rRT-PCR) assay termed CDC2022, which targets sequences encoding conserved amino acid regions of all extant EV-D68 strains. We targeted three motifs conserved among all strains in the last 60 years. The assay achieved 100% (281/281) sensitivity and 100% (344/344) specificity when tested with a collection of 625 respiratory specimens, compared to the gold-standard EV semi-nested VP1 PCR and sequencing assay (snPCR/Seq). CDC2022 gave negative results with 289/289 non-target viruses, including 104 EV A-D isolates, 165 rhinovirus (RV) isolates or clinical specimens, and 14 other common respiratory viruses. The limit of detection (LOD) of the CDC2022 assay is 361 copies per reaction, as determined using serially diluted RNA transcripts. It can detect as few as 0.28 CCID50 per reaction with titrated isolates. An in silico "phylo-primer-mismatch" analysis was performed to visualize primer/probe mismatches and to compare CDC2022 with other EV-D68 rRT-PCR assays. It showed that CDC2022 has the fewest primer/probe mismatches among all assays analyzed and is suitable for all clades. As a type-specific assay targeting conserved amino acids, the CDC2022 assay allowed detection of newer strains from 2024. The CDC 2022 assay could provide a critical tool for molecular surveillance of EV-D68.IMPORTANCEEV-D68 has caused recurring respiratory disease outbreaks in the United States since 2014. As recurrent outbreaks and continued virus evolution are expected for EV-D68, the CDC2022 rRT-PCR provides a robust test that detects known strains as well as potential emerging strains. This type-specific assay approach is critical for national EV-D68 surveillance and clinical diagnostics. An in silico "phylo-primer-mismatch" approach is invented to show EV-D68 assay robustness, but it has utility in new molecular tests for pathogen detection.
Keywords: EV-D68; Enterovirus D68; diagnostic assay; rRT-PCR; real-time PCR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mishra N, Ng TFF, Marine RL, Jain K, Ng J, Thakkar R, Caciula A, Price A, Garcia JA, Burns JC, Thakur KT, Hetzler KL, Routh JA, Konopka-Anstadt JL, Nix WA, Tokarz R, Briese T, Oberste MS, Lipkin WI. 2019. Antibodies to enteroviruses in cerebrospinal fluid of patients with acute flaccid myelitis. mBio 10:e01903-19. doi: 10.1128/mBio.01903-19 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

