A β-cap on the FliPQR protein-export channel acts as the cap for initial flagellar rod assembly
- PMID: 40833400
- PMCID: PMC12403130
- DOI: 10.1073/pnas.2507221122
A β-cap on the FliPQR protein-export channel acts as the cap for initial flagellar rod assembly
Abstract
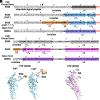
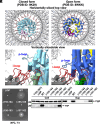
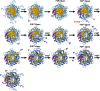
The FliPQR complex constitutes a channel for export of the flagellar proteins involved in axial structure assembly. It also serves as a template for the assembly of the rod structure, which consists of FliE, FlgB, FlgC, FlgF, and FlgG. FliP, FliQ, and FliR assemble into a right-handed helical structure within the central pore of the flagellar basal body MS-ring, and the complex has two gates on the cytoplasmic and periplasmic sides. The periplasmic gate, formed by the N-terminal α-helices of FliP and FliR, remains closed until six FliE subunits assemble onto FliP and FliR to form the first layer of the rod, but it has remained unclear how each FliE subunit opens the gate and assembles in the absence of the rod cap required for efficient assembly of other rod proteins. Here, we present a cryoelectron microscopy structure of the FliPQR complex in closed form at 3.0 Å resolution. A β-cap, formed by the N-terminal β-strands of FliP and FliR, is located at the top of the FliPQR complex and tightly seals the closed gate. The β-cap has a narrow pore that efficiently and accurately leads the first FliE subunit to its assembly site. Interactions of FliE with FliP and FliR induce a conformational change in FliP and FliR, with their N-terminal α-helices move up and outward to open the gate. Consequently, each of the N-terminal β-strands of FliP and FliR detaches from the β-cap one after another, thereby creating a docking site for the next FliE subunit to efficiently assemble.
Keywords: cryoelectron microscopy; flagellar rod; flagellum; type III secretion system.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




Similar articles
-
Genetic Analysis of the Salmonella FliE Protein That Forms the Base of the Flagellar Axial Structure.mBio. 2021 Oct 26;12(5):e0239221. doi: 10.1128/mBio.02392-21. Epub 2021 Sep 28. mBio. 2021. PMID: 34579566 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
FliO is an evolutionarily conserved yet diversified core component of the bacterial flagellar type III secretion system.Proc Natl Acad Sci U S A. 2025 Aug 26;122(34):e2512476122. doi: 10.1073/pnas.2512476122. Epub 2025 Aug 21. Proc Natl Acad Sci U S A. 2025. PMID: 40838884 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff.Cochrane Database Syst Rev. 2016 Apr 19;4:CD011621. doi: 10.1002/14651858.CD011621.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2019 Jul 01;7:CD011621. doi: 10.1002/14651858.CD011621.pub3. PMID: 27093058 Free PMC article. Updated.
References
MeSH terms
Substances
Grants and funding
- JP20K15749/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP22K06162/MEXT | Japan Society for the Promotion of Science (JSPS)
- 19H03182/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP22H02573/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP20H05532/Ministry of Education, Culture, Sports, Science and Technology (MEXT)
LinkOut - more resources
Full Text Sources
Miscellaneous

