Monoamine-induced diacylglycerol signaling rapidly accumulates Unc13 in nanoclusters for fast presynaptic potentiation
- PMID: 40833403
- PMCID: PMC12403152
- DOI: 10.1073/pnas.2514151122
Monoamine-induced diacylglycerol signaling rapidly accumulates Unc13 in nanoclusters for fast presynaptic potentiation
Abstract
Neuromodulators control mood, arousal, and behavior by inducing synaptic plasticity via G-protein-coupled receptors. While long-term presynaptic potentiation requires structural changes, mechanisms enabling potentiation within minutes remain unclear. Using the Drosophila neuromuscular junction, we show that octopamine, the invertebrate analog of norepinephrine, potentiates evoked neurotransmitter release on the timescale of one minute via a G-protein-coupled pathway involving presynaptic OAMB receptors and phospholipase C. This fast potentiation correlates with elevated signals of the release factor Unc13A and the scaffolding protein Bruchpilot. Live, single-molecule imaging of endogenously tagged Unc13 revealed its instantly reduced motility and increased concentration in synaptic nanoclusters with potentiation. Presynaptic knockdown of Unc13A fully blocked fast potentiation. Moreover, deleting its N-terminal localization sequence mislocalized the protein fragment to the cytosol, but still allowed for rapid plasma membrane recruitment by diacylglycerol (DAG) analog phorbol esters and octopamine, implicating a role of more C-terminal domains. A point mutation of endogenous Unc13 in its DAG-binding C1 domain blocked plasticity-induced nanoscopic enrichment and synaptic potentiation. The mutation increased basal neurotransmission but reduced Unc13 levels, revealing a gain of function and potential homeostatic compensation. The mutation also blocked phorbol ester-induced potentiation, decreased the calcium sensitivity of neurotransmission, and caused short-term synaptic depression. Homeostatic potentiation induced by postsynaptic receptor block mirrored octopamine-induced Unc13 recruitment and required presynaptic OAMB receptors, indicating overlapping machinery. Thus, rapid Unc13 immobilization and nanoscale compaction are salient features of fast presynaptic potentiation.
Keywords: G-protein- coupled receptors; Munc13; Neuromodulation; neurotransmitter release; synaptic plasticity.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

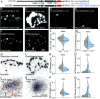


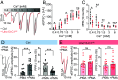


Similar articles
-
Rapid active zone remodeling consolidates presynaptic potentiation.Nat Commun. 2019 Mar 6;10(1):1085. doi: 10.1038/s41467-019-08977-6. Nat Commun. 2019. PMID: 30842428 Free PMC article.
-
The Roles of the Numb Protein in Synaptic Development and Plasticity.Dev Neurobiol. 2025 Jul;85(3):e22988. doi: 10.1002/dneu.22988. Dev Neurobiol. 2025. PMID: 40620134
-
Layered reward signalling through octopamine and dopamine in Drosophila.Nature. 2012 Dec 20;492(7429):433-7. doi: 10.1038/nature11614. Epub 2012 Oct 28. Nature. 2012. PMID: 23103875 Free PMC article.
-
Regulation of presynaptic homeostatic plasticity by glial signalling in Alzheimer's disease.J Physiol. 2024 Dec 20:10.1113/JP286751. doi: 10.1113/JP286751. Online ahead of print. J Physiol. 2024. PMID: 39705214 Review.
-
Adrenergic Modulation of Acetylcholine Release at the Mouse Neuromuscular Junctions of Fast-Twitch Skeletal Muscle.Neurochem Res. 2025 May 12;50(3):162. doi: 10.1007/s11064-025-04415-w. Neurochem Res. 2025. PMID: 40353941 Review.
References
-
- Walter A. M., Bohme M. A., Sigrist S. J., Vesicle release site organization at synaptic active zones. Neurosci. Res. 127, 3–13 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

