Evaluating the association between migraine treatments and tinnitus: Insights from the US Food and Drug Administration adverse event reporting system
- PMID: 40833961
- PMCID: PMC12367135
- DOI: 10.1371/journal.pone.0330493
Evaluating the association between migraine treatments and tinnitus: Insights from the US Food and Drug Administration adverse event reporting system
Abstract
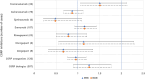
Tinnitus, a distressing condition that can significantly impair quality of life, has been associated with several medications, including triptans. This study aimed to explore the relationship between tinnitus and specific migraine treatments, focusing on triptans and calcitonin gene-related peptide (CGRP) inhibitors. Data from the FDA Adverse Event Reporting System (FAERS) through the third quarter of 2023 were analyzed to calculate proportional reporting ratios (PRR) and reporting odds ratios (ROR) for migraine treatments, specifically triptans and CGRP inhibitors. Positive tinnitus signals were identified when PRRs or RORs were greater than 2.0, the lower bound of the 95% confidence interval exceeded 1.0, and at least three cases were reported. Intra- and inter-class analyses were conducted to compare tinnitus reports among individual drugs and drug classes. Among 47,615 tinnitus-related adverse events, 345 were associated with CGRP inhibitors and 183 with triptans. Positive tinnitus signals were observed for several CGRP inhibitors, except eptinezumab and atogepant, and for all triptans except sumatriptan. Inter-class analysis revealed no significant differences between triptans and CGRP inhibitors. However, intra-class analysis identified naratriptan, almotriptan, and frovatriptan as having notable tinnitus signals among triptans, while CGRP inhibitors did not exhibit strong signals for any specific drug. Using real-world data from FAERS and pharmacovigilance methods, this study identified tinnitus signals related to migraine treatments, particularly among certain triptans. These findings provide preliminary evidence for further investigation into the relationship between migraine medications and tinnitus.
Copyright: © 2025 Kim et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Hoffman HJ, Reed GW. Epidemiology of tinnitus. In: Snow JB, editor. Tinnitus: Theory and Management. Lewiston, NY: BC Decker; 2004. pp. 16–41.
-
- Bauer CA. Tinnitus. N Engl J Med. 2018;378(13):1224–31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

