Human-induced pluripotent stem cell-based hepatic modeling of lipid metabolism-associated TM6SF2-E167K variant
- PMID: 40833996
- PMCID: PMC11865362
- DOI: 10.1097/HEP.0000000000001065
Human-induced pluripotent stem cell-based hepatic modeling of lipid metabolism-associated TM6SF2-E167K variant
Abstract
Background and aims: TM6SF2 rs58542926 (E167K) is related to an increased prevalence of metabolic dysfunction-associated steatotic liver disease. Conflicting mouse study results highlight the need for a human model to understand this mutation's impact. This study aims to create and characterize a reliable human in vitro model to mimic the effects of the TM6SF2-E167K mutation for future studies.
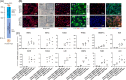
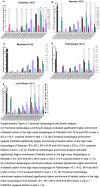
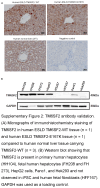
Approach and results: We used gene editing on human-induced pluripotent stem cells from a healthy individual to create cells with the TM6SF2-E167K mutation. After hepatocyte-directed differentiation, we observed decreased TM6SF2 protein expression, increased intracellular lipid droplets, and total cholesterol, in addition to reduced VLDL secretion. Transcriptomics revealed the upregulation of genes involved in lipid, fatty acid, and cholesterol transport, flux, and oxidation. Global lipidomics showed increased lipid classes associated with endoplasmic reticulum (ER) stress, mitochondrial dysfunction, apoptosis, and lipid metabolism. In addition, the TM6SF2-E167K mutation conferred a proinflammatory phenotype with signs of mitochondria and ER stress. Importantly, by facilitating protein folding within the ER of hepatocytes carrying TM6SF2-E167K mutation, VLDL secretion and ER stress markers improved.
Conclusions: Our findings indicate that induced hepatocytes generated from human-induced pluripotent stem cells carrying the TM6SF2-E167K recapitulate the effects observed in human hepatocytes from individuals with the TM6SF2 mutation. This study characterizes an in vitro model that can be used as a platform to identify potential clinical targets and highlights the therapeutic potential of targeting protein misfolding to alleviate ER stress and mitigate the detrimental effects of the TM6SF2-E167K mutation on hepatic lipid metabolism.
Keywords: TM6SF2 E167K; human hepatocytes; iPSC; lipid metabolism; liver disease model.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Alina Ostrowska owns stock in Pittsburgh ReLiver Inc. Ira J. Fox owns stocks in Pittsburgh ReLiver Inc. Alejandro Soto-Gutierrez owns stock in VonBaer Wolff Inc and Pittsburgh ReLiver Inc. The remaining authors have no conflicts to report.
Figures










Update of
-
Human Induced Pluripotent Stem Cell based Hepatic-Modeling of Lipid metabolism associated TM6SF2 E167K variant.bioRxiv [Preprint]. 2023 Dec 18:2023.12.18.572248. doi: 10.1101/2023.12.18.572248. bioRxiv. 2023. Update in: Hepatology. 2025 Sep 1;82(3):638-654. doi: 10.1097/HEP.0000000000001065. PMID: 38187603 Free PMC article. Updated. Preprint.
Similar articles
-
Human Induced Pluripotent Stem Cell based Hepatic-Modeling of Lipid metabolism associated TM6SF2 E167K variant.bioRxiv [Preprint]. 2023 Dec 18:2023.12.18.572248. doi: 10.1101/2023.12.18.572248. bioRxiv. 2023. Update in: Hepatology. 2025 Sep 1;82(3):638-654. doi: 10.1097/HEP.0000000000001065. PMID: 38187603 Free PMC article. Updated. Preprint.
-
Basic and translational evidence supporting the role of TM6SF2 in VLDL metabolism.Curr Opin Lipidol. 2024 Jun 1;35(3):157-161. doi: 10.1097/MOL.0000000000000930. Epub 2024 Mar 8. Curr Opin Lipidol. 2024. PMID: 38465912 Free PMC article. Review.
-
Therapeutic effects of adipose tissue-derived mesenchymal stem cells on ER stress in a murine model of metabolic dysfunction-associated steatohepatitis: an in vivo and in vitro study.Stem Cell Res Ther. 2025 Jul 6;16(1):349. doi: 10.1186/s13287-025-04482-4. Stem Cell Res Ther. 2025. PMID: 40619419 Free PMC article.
-
TM6SF2 Determines Both the Degree of Lipidation and the Number of VLDL Particles Secreted by the Liver.medRxiv [Preprint]. 2023 Jun 29:2023.06.23.23291823. doi: 10.1101/2023.06.23.23291823. medRxiv. 2023. PMID: 37425717 Free PMC article. Preprint.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
References
-
- Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. - PubMed
MeSH terms
Substances
Grants and funding
- U2C TR004863/TR/NCATS NIH HHS/United States
- U01 TR002383/TR/NCATS NIH HHS/United States
- UH3 DK119973/DK/NIDDK NIH HHS/United States
- P30 DK120531/DK/NIDDK NIH HHS/United States
- P01 DK096990/DK/NIDDK NIH HHS/United States
- UG3 DK119973/DK/NIDDK NIH HHS/United States
- R01 DK099257/DK/NIDDK NIH HHS/United States
- R01 DK117881/DK/NIDDK NIH HHS/United States
- R01 DK135606/DK/NIDDK NIH HHS/United States
- UG3 TR003289/TR/NCATS NIH HHS/United States
- S10 OD028450/OD/NIH HHS/United States
- UH3 TR003289/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources

