Leukemic Cells Hijack Stromal Bioelectricity to Reprogram the Bone Marrow Niche via CaV1.2-Dependent Mechanisms
- PMID: 40834435
- PMCID: PMC12533209
- DOI: 10.1002/advs.202508940
Leukemic Cells Hijack Stromal Bioelectricity to Reprogram the Bone Marrow Niche via CaV1.2-Dependent Mechanisms
Abstract
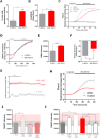
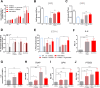
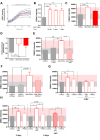
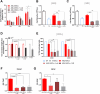
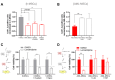
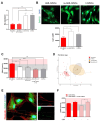
Mesenchymal stromal cells (MSCs) are key components of the tumor microenvironment (TME), influencing leukemia progression through poorly understood mechanisms. Here, the bioelectrical properties of MSCs derived from pediatric acute myeloid leukemia (AML) patients (AML-MSCs) are investigated, identifying a significant depolarization of their resting voltage membrane potential (Vmem, -14.7 mV) compared to healthy MSCs (h-MSCs, -28.5 mV), accompanied by downregulation of Calcium channel, voltage-dependent, L type, alpha 1C subunit1.2 (CaV1.2) L-type calcium channel expression. AML-MSCs display increased spontaneous calcium oscillations, suggesting altered ion homeostasis. Notably, h-MSCs exposed to AML blasts undergo a similar Vmem depolarization (-11.8 mV) and CaV1.2 downregulation, indicating that leukemic cells actively reprogram MSCs. Functionally, Vmem depolarization in h-MSCs promotes a pro-leukemic phenotype, whereas hyperpolarization of AML-MSCs restores a normal behavior. CaV1.2 over-expression by lentiviral vectors in AML-MSCs shifts the Vmem toward hyperpolarization and partially reverses their leukemia-supportive properties, in part through CaV1.2 transfer via tunneling nanotubes. These findings reveal that AML blasts impose a bioelectrical signature on MSCs, modulating ion channel activity to sustain a leukemic niche. Targeting this electrical reprogramming through CaV1.2 restoration represents a potential strategy to re-establish homeostasis in the bone marrow microenvironment.
Keywords: acute myeloid leukemia; bioelectricity; mesenchymal stromal cells; tumor microenvironment; voltage membrane potential.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Hoggatt J., Kfoury Y., Scadden D. T., Annu. Rev. Pathol. Mech. Dis. 2016, 11, 555. - PubMed
-
- Fan J. J., Huang X.. in Reviews of physiology, biochemistry and pharmacology, Springer International Publishing, Berlin: 2020, pp. 103–133.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
