Longitudinal association of shorter leukocyte telomere length with CSF biomarker dynamics across early Alzheimer's disease stages in at-risk individuals
- PMID: 40834628
- PMCID: PMC12395440
- DOI: 10.1016/j.ebiom.2025.105886
Longitudinal association of shorter leukocyte telomere length with CSF biomarker dynamics across early Alzheimer's disease stages in at-risk individuals
Abstract
Background: Short telomere length (TL), a hallmark of biological ageing, has been associated with an increased risk of Alzheimer's disease (AD), but its pathophysiological role remains unclear. This study explored the relationship between blood leukocyte TL (LTL), cerebrospinal fluid (CSF) AD biomarkers changes, and brain structure across early stages of the AD continuum.
Methods: We included 346 cognitively unimpaired participants (aged 49-71) from the ALFA cohort, enriched for AD risk (53.2% APOE-ε4 carriers; 34% amyloid-positive). LTL was measured at baseline (visit 0) using quantitative PCR. Associations were assessed between baseline LTL and CSF biomarkers at visit 1 (mean follow-up from baseline = 3.98 years, SD = 1.02), and with changes in CSF biomarkers between visits 1 and 2 (mean interval = 3.45 years, SD = 0.58). Cortical thickness in ageing- and AD-vulnerable brain regions was evaluated by magnetic resonance imaging (MRI) at visit 1. Analyses were stratified by APOE-ε4 status and amyloid-tau (AT) profiles. Mediation models tested whether CSF biomarkers mediated LTL-cortical thickness associations.
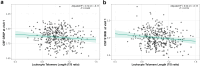
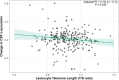
Findings: Shorter LTL was associated with higher astrocytic reactivity at visit 1 and with increased synaptic dysfunction over time. Among APOE-ε4 carriers and AT-positive individuals, shorter LTL was associated with higher p-tau181 and neurodegeneration markers. Shorter LTL was associated with greater cortical thickness in ageing- and AD-vulnerable regions, partially mediated by astrocytic reactivity biomarkers.
Interpretation: These findings suggest that shorter telomeres are associated with early AD-related biological changes, potentially via mechanisms involving astrocytic reactivity and brain structural alterations. LTL may serve as an early marker of vulnerability to neurodegenerative processes in at-risk populations.
Funding: AARG-19-618265; PI19/00119; LCF/PR/GN17/10300004; TriBEKa-17-519007; # SLT002/16/00201.
Keywords: Alzheimer's disease; Cortical thickness; Glial biomarkers; Leukocyte telomere length; Preclinical.
Copyright © 2025 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests BR-F, AG-E, TEE, MS-C, PO-R, NA, AN, and AS-V have nothing to disclose. GK is a full-time employee of Roche Diagnostics GmbH. CM has received research funding from an EU-FINGERS JPND research grant and from an ADDF digital biomarkers research grant; both paid to the institution. JDG receives research funding from Roche Diagnostics, Hoffmann-La Roche, GE Healthcare, the Innovative Health Initiative (IHI) of the European Commission (Grant agreement No. 101112145), BrightFocus Foundation (A2022034S), Instituto de Salud Carlos III (PMP22/00022), and Fundació La Marató de TV3 (202318-30-31-32). He has given lectures in symposia sponsored by Biogen, Philips, and Life-MI; received consulting fees from Roche Diagnostics; and serves on a scientific advisory board at Prothena Biosciences. HZ has served on scientific advisory boards and/or as a consultant for: Abbvie, Acumen, Alector, Alzinova, ALZpath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Quanterix, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave. He has given lectures sponsored by: Alzecure, BioArctic, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, Roche, and WebMD. HZ is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is part of the GU Ventures Incubator Program (outside the submitted work). He is supported by the Swedish Research Council (#2023-00356, #2022-01018, and #2019-02397); the European Union's Horizon Europe research and innovation programme (grant agreement No. 101053962); Swedish State Support for Clinical Research (#ALFGBG-71320); Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862); the AD Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21-831381-C, #ADSF-21-831377-C, and #ADSF-24-1284328-C); the European Partnership on Metrology (NEuroBioStand, #22HLT07); the Bluefield Project; Cure Alzheimer's Fund; the Olav Thon Foundation; the Erling-Persson Family Foundation; Familjen Rönströms Stiftelse; Stiftelsen för Gamla Tjänarinnor; Hjärnfonden, Sweden (#FO2022-0270); the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 860197 (MIRIADE); the European Union Joint Programme—Neurodegenerative Disease Research (JPND2021-00694); the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre; and the UK Dementia Research Institute at UCL (UKDRI-1003). KB has served as a consultant, on advisory boards, or on data monitoring committees for: Abcam, Axon, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Prothena, Roche Diagnostics, and Siemens Healthineers. He is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), part of the GU Ventures Incubator Program. He is supported by the Swedish Research Council (#201700915); Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB2018092016615); the Swedish Alzheimer Foundation (#AF742881); Hjärnfonden, Sweden (#FO20170243); the Swedish state under the agreement between the Swedish government and the County Councils, the ALF agreement (#ALFGBG715986); the European Union Joint Program for Neurodegenerative Disorders (JPND2019466236); the National Institutes of Health (NIH), USA (grant #1R01AG06839801); and the Alzheimer's Association 2021 Zenith Award (ZEN21848495). MS-C has received in the past 36 months consultancy/speaker fees (paid to the institution) from Almirall, Eli Lilly, Novo Nordisk, and Roche Diagnostics. He has received consultancy fees or served on advisory boards (paid to the institution) for Eli Lilly, Grifols, and Roche Diagnostics. He was granted a project and is a site investigator of a clinical trial (funded to the institution) by Roche Diagnostics. In-kind support for research (to the institution) was received from: ADx Neurosciences, Alamar Biosciences, ALZPath, Avid Radiopharmaceuticals, Eli Lilly, Fujirebio, Janssen Research & Development, Meso Scale Discovery, and Roche Diagnostics. MS-C did not receive any personal compensation from these organisations or any other for-profit organisation. MS-C receives funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (Grant agreement No. 948677); the Instituto de Salud Carlos III (ISCIII) through projects PI19/00155 and PI22/00456 (co-funded by European Regional Development Fund (FEDER) “A way to make Europe”) and receives support from a fellowship funded by “la Caixa” Foundation (ID 100010434), and the Marie Skłodowska-Curie grant agreement No. 847648 (fellowship code LCF/BQ/PR21/11840004). MC-B receives funding from the Alzheimer's Association International (Grant AARG-19–618265); and the Spanish Ministry of Health. NV-T has received funding from the Juan de la Cierva Incorporación program (IJDC2020-043216-I) and Ramón y Cajal (RYC2022-038136-I) programmes, funded by the Ministerio de Ciencia, Innovación y Universidades–Spanish State Research Agency (MCIN/AEI/10.13039/501100011033), co-funded by the European Union “Next GenerationEU”/PRTR, and project PID2022-143106OA-I00 (co-funded by the European Union FEDER). She receives additional funding from the Alzheimer's Disease Data Initiative (ADDI) through the William H. Gates Sr. Fellowship Program, from the Ajuntament de Barcelona and “la Caixa” Foundation (project 23S06083-001), and from Alzheimer Nederland (InterAct-beurs ′24, #WE.08-2024-07). BR-F and PG have received honoraria for lectures from the University of Vic-Central University of Catalonia, and travel support from the Alzheimer's Association International. NV-T has received honoraria for lectures from the University of Vic-Central University of Catalonia, the University of Valencia, and UNED. She has also received travel support from ADDI, Alzheimer Nederland, and the Alzheimer's Association International. She serves as scientific co-director of the PRISMA association and is on the board committee of the Spanish Biostatistics Society.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

