Changes in brain connectivity and neurovascular dynamics during dexmedetomidine-induced loss of consciousness
- PMID: 40835723
- PMCID: PMC12368020
- DOI: 10.1038/s42003-025-08577-9
Changes in brain connectivity and neurovascular dynamics during dexmedetomidine-induced loss of consciousness
Abstract
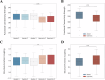
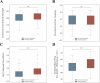
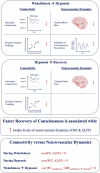
Understanding the neurophysiological changes underlying conscious-unconscious transitions is a key goal in neuroscience. Using magnetic resonance neuroimaging, we investigate the network connectivity and neurovascular changes occurring as the human brain transitions from wakefulness to dexmedetomidine-induced hypnosis, and recovery. Hypnosis led to widespread decreases in functional connectivity strength and increased structure-function coupling, indicating functional patterns more constrained by the underlying anatomical connectivity. As individuals began to regain consciousness, both connectivity markers returned towards awake levels, with particularly prominent coupling changes across the cerebellum. Neurovascular dynamics were disrupted during hypnosis as well: cerebral blood flow decreased globally-most notably in the brainstem, thalamus, and cerebellum-and continued decreasing even as recovery commenced, except within the cerebellum. Notably, regions with higher functional connectivity strength during wakefulness exhibited greater blood flow reductions during hypnosis. Hypnosis also heightened the amplitude of low-frequency fluctuations in the hemodynamic signal, especially in visual and somatomotor regions. Critically, individuals who regained consciousness faster displayed higher baseline levels of both neurovascular, but not connectivity, markers. Together, these results reveal that the induction of, and emergence from, dexmedetomidine-induced unconsciousness involve widespread, coordinated changes in brain connectivity and neurovascular function; across our findings, we also highlight the recurrent role of cerebellum in conscious-unconscious transitions.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Citation diversity statement: Recent work in several fields of science has identified a bias in citation practices such that papers from women and other minority scholars are under-cited relative to the number of such papers in the field105–109. We obtained the predicted gender of the first and last author of each reference by using databases that store the probability of a first name being carried by a woman109. By this measure (and excluding self-citations to the first and last authors of our current paper), our references contain 3% woman(first)/woman(last), 12.9% man/woman, 29.7% woman/man, and 54.4% man/man. This method is limited in that a) names, pronouns, and social media profiles used to construct the databases may not, in every case, be indicative of gender identity and b) it cannot account for intersex, non-binary, or transgender people. We look forward to future work that could help us better understand how to support equitable practices in science.
Figures





Update of
-
Changes in brain connectivity and neurovascular dynamics during dexmedetomidine-induced loss of consciousness.bioRxiv [Preprint]. 2024 Nov 12:2024.10.04.616650. doi: 10.1101/2024.10.04.616650. bioRxiv. 2024. Update in: Commun Biol. 2025 Aug 20;8(1):1254. doi: 10.1038/s42003-025-08577-9. PMID: 39416182 Free PMC article. Updated. Preprint.
Similar articles
-
Changes in brain connectivity and neurovascular dynamics during dexmedetomidine-induced loss of consciousness.bioRxiv [Preprint]. 2024 Nov 12:2024.10.04.616650. doi: 10.1101/2024.10.04.616650. bioRxiv. 2024. Update in: Commun Biol. 2025 Aug 20;8(1):1254. doi: 10.1038/s42003-025-08577-9. PMID: 39416182 Free PMC article. Updated. Preprint.
-
Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness.J Neurosci. 2012 May 16;32(20):7082-90. doi: 10.1523/JNEUROSCI.3769-11.2012. J Neurosci. 2012. PMID: 22593076 Free PMC article. Clinical Trial.
-
Wakefulness can be distinguished from general anesthesia and sleep in flies using a massive library of univariate time series analyses.PLoS Biol. 2025 Jul 10;23(7):e3003217. doi: 10.1371/journal.pbio.3003217. eCollection 2025 Jul. PLoS Biol. 2025. PMID: 40638578 Free PMC article.
-
Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients.Cochrane Database Syst Rev. 2015 Jan 6;1(1):CD010269. doi: 10.1002/14651858.CD010269.pub2. Cochrane Database Syst Rev. 2015. PMID: 25879090 Free PMC article.
-
Hypnosis for pain management during labour and childbirth.Cochrane Database Syst Rev. 2016 May 19;2016(5):CD009356. doi: 10.1002/14651858.CD009356.pub3. Cochrane Database Syst Rev. 2016. PMID: 27192949 Free PMC article.
References
-
- Shehabi, Y. et al. Early sedation with dexmedetomidine in critically ill patients. N. Engl. J. Med.380, 2506–2517 (2019). - PubMed
-
- Huupponen, E. et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol. Scand.52, 289–294 (2008). - PubMed
MeSH terms
Substances
Grants and funding
- UL1RR024134/U.S. Department of Health & Human Services | NIH | National Center for Research Resources (NCRR)
- RF1 MH116920/MH/NIMH NIH HHS/United States
- R01 EB031080/EB/NIBIB NIH HHS/United States
- T32EB020087/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 MH113550/MH/NIMH NIH HHS/United States
- R35 GM142712/GM/NIGMS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- RF1MH116920/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01NS112274/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R21MH106799/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R35GM142712/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 NS112274/NS/NINDS NIH HHS/United States
- R01 MH112847/MH/NIMH NIH HHS/United States
- R01MH123550/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01MH113550/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01MH112847/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R37 MH125829/MH/NIMH NIH HHS/United States
- R01 MH123550/MH/NIMH NIH HHS/United States
- R01GM088156/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R37MH125829/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R21 MH106799/MH/NIMH NIH HHS/United States
- UL1TR000003/U.S. Department of Health & Human Services | NIH | National Center for Advancing Translational Sciences (NCATS)
- T32 EB020087/EB/NIBIB NIH HHS/United States
- R01EB031080/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01GM144377/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01 GM144377/GM/NIGMS NIH HHS/United States
- R01 GM088156/GM/NIGMS NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

