Highly stable and efficient copper(I) sensitizer for narrowband red organic light-emitting diodes with an operational lifetime (LT95) of up to 3689 h at 1000 cd m-2
- PMID: 40835784
- PMCID: PMC12368058
- DOI: 10.1038/s41467-025-62867-8
Highly stable and efficient copper(I) sensitizer for narrowband red organic light-emitting diodes with an operational lifetime (LT95) of up to 3689 h at 1000 cd m-2
Abstract
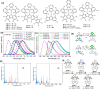
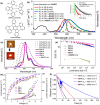
Copper-based organic light-emitting diodes (OLEDs) are low-cost alternatives to precious metal-based OLEDs, but currently no such OLEDs can meet the practical requirements for high colour purity, device efficiency, and operational stability. Carbene-Cu(I)-amide emitters reported here exhibited thermally activated delayed fluorescent emission with quantum efficiencies up to 0.90 and radiative decay rates of 2.7 × 106 s-1. These enable blue to near-infrared Cu(I)-OLEDs with high brightness (265,000 cd m-2) and extended LT95 lifetime (3582 hours at 1000 cd m-2). Deuteriation and π-extension of carbazole significantly enhance OLED stability. Cu(I)-sensitized fluorescence OLEDs showed efficient narrowband electroluminescence (λmax 612-614 nm; full-width half maximum of 33-38 nm; maximum external quantum efficiencies reach 21.9%) and prolonged LT95 lifetime (up to 3689 h at 1000 cd m-2). This work highlights earth-abundant metal-based sensitized-OLEDs that exhibit high colour purity and long device lifetime comparable to the best non-iridium metal-based OLEDs.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: HKU has filed patent applications on part of the materials in this work. C.-M.C., R.T., and G.C. are the authors of the invention. Patent applications No. US18/057,632 (Pending), JP2024530039A (Pending), EP22897837.5 A (Pending), KR1020247019615A (Pending), CN202211477600.8 A (Pending) and PCT/CN2022/133671 (Application Filing) and US63/859,537 (Pending). The other authors declare no competing interests.
Figures


References
-
- Wei, Q. et al. Small-Molecule Emitters with High Quantum Efficiency: Mechanisms, Structures, and Applications in OLED Devices. Adv. Opt. Mater.6, 1800512 (2018).
-
- Hong, G. et al. A Brief History of OLEDs — Emitter Development and Industry Milestones. Adv. Mater.33, 2005630 (2021). - PubMed
-
- Tang, M.-C. et al. Molecular Design of Luminescent Gold(III) Emitters as Thermally Evaporable and Solution-Processable Organic Light-Emitting Device (OLED) Materials. Chem. Rev.121, 7249–7279 (2021). - PubMed
-
- Kim, J.-M. et al. Tetradentate Pt complexes for organic light-emitting diodes. Trends Chem.5, 267–278 (2023).
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

