Identification and preclinical efficacy evaluation of two lytic bacteriophages targeting highly virulent and multidrug-resistant Klebsiella pneumoniae
- PMID: 40835923
- PMCID: PMC12369090
- DOI: 10.1186/s12941-025-00812-9
Identification and preclinical efficacy evaluation of two lytic bacteriophages targeting highly virulent and multidrug-resistant Klebsiella pneumoniae
Abstract
Background: The emergence of MDR K. pneumoniae poses a critical challenge in treating respiratory-associated pneumonia. Bacteriophages are promising antibiotic alternatives with unique features. This study aimed to isolate new bacteriophages from the hospital environment and investigate their therapeutic potential and mechanisms.
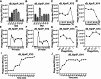
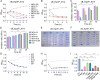
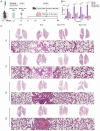
Methods: We employed plaque assays, transmission electron microscopy, and whole-genome sequencing to systematically characterize the biological properties, morphology, and genomic profiles of the phages in parallel. The bacteriostatic curve, biofilm staining quantification, and biofilm inhibition rate assay were employed to evaluate the in vitro lytic efficacy of the phage. More importantly, we established the murine pneumonia infection models through nasal instillation, assessed the therapeutic potential of the phage in vivo by observing pathological morphology via HE staining, detecting pro-inflammatory cytokine levels via qPCR and ELISA, and monitoring bacterial load changes in lung tissue through PCR analysis.
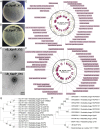
Results: Phages vB_KpnP_XY3 and vB_KpnP_XY4, taxonomically classified as Siphoviridae, demonstrated broad temperature (4-60 °C), pH (4-11) tolerance, chloroform resistance, latent periods of 40/35 min, and burst sizes of 340/126 PFU/cell. Both genomes contained circular dsDNA genomes (47,466 bp/50,036 bp) without virulence or antibiotic resistance genes. The bacterial concentration markedly decreased at 2 h post-treatment, reaching its biological nadir by 6 h. Concurrent biofilm assays demonstrated 80% biofilm inhibition and rapid bacterial clearance. In murine pneumonia models, both phage monotherapy and phage-antibiotic combinations significantly reduced bacterial loads compared with antibiotics alone (P < 0.05), concurrently attenuating inflammation (IL-1β/IL-6/TNF-a. P < 0.0001) and restoring alveolar architecture with reduced necrosis.
Conclusion: The phages vB_KpnP_XY3 and vB_KpnP_XY4 demonstrated robust environmental adaptability. Its antibacterial effect is related to its specific biofilm dissolution performance in vivo and in vitro. These findings provide strong evidence for the precise phage treatment of MDR K. pneumoniae infections.
Keywords: Bacteriophage therapy; Biofilm degradation; Histopathology; Lytic bacteriophage; Multidrug-resistant Klebsiella pneumoniae.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Ethical Committee for Animal Experimentation of Xiangyang No. 1 People’s Hospital (approval number XYYYE20240114). Clinical trial number: not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Isolation and characterization of phages ΦZC2 and ΦZC3 against carbapenem-resistant Acinetobacter baumannii, and efficacy of ΦZC3 on A549 cells.Virol J. 2025 Jul 30;22(1):262. doi: 10.1186/s12985-025-02885-6. Virol J. 2025. PMID: 40739242 Free PMC article.
-
Characterization and antimicrobial activity of a novel lytic phage vB_SmaS_QH16 against Stenotrophomonas maltophilia: in vitro, in vivo, and biofilm studies.Front Cell Infect Microbiol. 2025 Jul 10;15:1610857. doi: 10.3389/fcimb.2025.1610857. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40708751 Free PMC article.
-
Lytic bacteriophages targeting multidrug-resistant Pseudomonas aeruginosa in Moschus berezovskii: isolation, characterization, and therapeutic efficacy against bacteremia.Virol J. 2025 Aug 19;22(1):285. doi: 10.1186/s12985-025-02715-9. Virol J. 2025. PMID: 40830499 Free PMC article.
-
Phage-encoded depolymerases and endolysins as prospective strategies to combat multidrug-resistant Klebsiella pneumoniae.Int J Biol Macromol. 2025 Sep;321(Pt 2):146159. doi: 10.1016/j.ijbiomac.2025.146159. Epub 2025 Jul 20. Int J Biol Macromol. 2025. PMID: 40695432 Review.
-
Prevalence and features of hypervirulent Klebsiella pneumoniae in respiratory specimens at a US hospital system.Infect Immun. 2025 Jan 31;93(1):e0048624. doi: 10.1128/iai.00486-24. Epub 2024 Dec 11. Infect Immun. 2025. PMID: 39660916 Free PMC article. Review.
References
-
- Holt KE, Wertheim H, Zadoks RN, Baker S, Whitehouse CA, Dance D et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proceedings of the National Academy of Sciences. 2015.112(27). - PMC - PubMed
MeSH terms
Grants and funding
- WJ2023M161/the Foundation of Health Commission of Hubei Province
- 2023KF001/the Open Research Fund Program of the State Key Laboratory of Virology of China at Wuhan University
- YC2024017/the Innovative Research Program for Graduate of Hubei University of Medicine
- 202500613/the Hubei Province Natural Science Foundation Joint Fund Project
- XYY2025LJ05/the Innovative Research Program of Xiangyang No.1 People's Hospital
LinkOut - more resources
Full Text Sources
Research Materials

