AI-based diagnosis of acute aortic syndrome from noncontrast CT
- PMID: 40835970
- PMCID: PMC12618251
- DOI: 10.1038/s41591-025-03916-z
AI-based diagnosis of acute aortic syndrome from noncontrast CT
Abstract
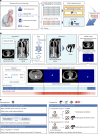
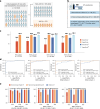
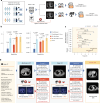
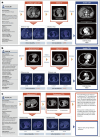
The accurate and timely diagnosis of acute aortic syndrome (AAS) in patients presenting with acute chest pain remains a clinical challenge. Aortic computed tomography (CT) angiography is the imaging protocol of choice in patients with suspected AAS. However, due to economic and workflow constraints in China, the majority of suspected patients initially undergo noncontrast CT as the initial imaging testing, and CT angiography is reserved for those at higher risk. Although noncontrast CT can reveal specific signs indicative of AAS, its diagnostic efficacy when used alone has not been well characterized. Here we present an artificial intelligence-based warning system, iAorta, using noncontrast CT for AAS identification in China, which demonstrates remarkably high accuracy and provides clinicians with interpretable warnings. iAorta was evaluated through a comprehensive step-wise study. In the multicenter retrospective study (n = 20,750), iAorta achieved a mean area under the receiver operating curve of 0.958 (95% confidence interval 0.950-0.967). In the large-scale real-world study (n = 137,525), iAorta demonstrated consistently high performance across various noncontrast CT protocols, achieving a sensitivity of 0.913-0.942 and a specificity of 0.991-0.993. In the prospective comparative study (n = 13,846), iAorta demonstrated the capability to significantly shorten the time to correct diagnostic pathway for patients with initial false suspicion from an average of 219.7 (115-325) min to 61.6 (43-89) min. Furthermore, for the prospective pilot deployment that we conducted, iAorta correctly identified 21 out of 22 patients with AAS among 15,584 consecutive patients presenting with acute chest pain and under noncontrast CT protocol in the emergency department. For these 21 AAS-positive patients, the average time to diagnosis was 102.1 (75-133) min. Finally, iAorta may help prevent delayed or missed diagnoses of AAS in settings where noncontrast CT remains the only feasible initial imaging modality-such as in resource-limited regions or in patients who cannot receive, or did not receive, intravenous contrast.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Alibaba Group has filed for patent protection (application number CN 202311181343.8) on behalf of Y.-J.Z., M.X. and L. Lu for the work related to the methods of detection of acute aortic syndrome on noncontrast CT. Y.-J.Z., W.G., J.Z., T.C.W.M., Z.L., L.L. and M.X. are employees of Alibaba Group and own Alibaba stock as part of the standard compensation package. All other authors have no competing interests.
Figures






References
-
- Mazzolai, L. et al. 2024 ESC guidelines for the management of peripheral arterial and aortic diseases. Eur. Heart J.45, 3538–3700 (2024). - PubMed
-
- Hagan, P. G. et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA283, 897–903 (2000). - PubMed
-
- Harris, K. M. et al. Correlates of delayed recognition and treatment of acute type A aortic dissection: the IRAD experience. Circulation124, 1911–1918 (2011). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

