Impact of PM2.5 exposure on acute toxicities and survival outcomes in radiotherapy for cervical cancer
- PMID: 40835997
- PMCID: PMC12368036
- DOI: 10.1038/s41598-025-15852-6
Impact of PM2.5 exposure on acute toxicities and survival outcomes in radiotherapy for cervical cancer
Abstract
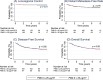
Particulate matter with a diameter less than 2.5 micrometers (PM2.5) remains a persistent environmental challenge in upper Northern Thailand. This study aimed to investigate whether exposure to PM2.5 during radiotherapy influences treatment-related acute toxicities and survival outcomes in cervical cancer patients. We retrospectively reviewed data from 411 cervical cancer patients diagnosed between 2013 and 2018 at Maharaj Nakorn Chiang Mai Hospital. Clinical data were obtained from medical records and the Chiang Mai Cancer Registry. Mean daily PM2.5 exposure during radiotherapy period was estimated using the data from the Copernicus Atmosphere Monitoring Service. Acute toxicity parameters, including gastrointestinal (GI), genitourinary (GU), and hematologic (anemia, leukopenia, and thrombocytopenia) toxicities, were analyzed using chi-square tests and logistic regression models. Survival outcomes-including locoregional control, distant metastasis, disease-free survival, and overall survival-were estimated using Kaplan-Meier curves and Cox proportional hazards models. Most patients (90%) had locally advanced disease, and nearly 80% received brachytherapy and chemotherapy. Patients exposed to mean daily PM2.5 levels above 25 [Formula: see text] had a significantly increased risk of developing GU toxicity (adjusted odds ratio 2.4; 95% confidence interval 1.3-4.2; p < 0.01). In contrast, PM2.5 exposure was not significantly associated with GI or hematologic toxicities, nor with differences in locoregional control, distant metastasis, disease-free survival, or overall survival. These findings suggest that daily PM2.5 exposure more than 25 [Formula: see text] is associated with a higher incidence of acute GU toxicity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: This study was approved by the Research Ethics Committee No.4, Faculty of Medicine, Chiang Mai University (Approval No. 200/2021), which also waived the requirement for informed consent due to the retrospective nature of the study and use of de-identified patient data. This study was carried out in accordance with the Helsinki Declaration.
Figures
References
-
- (WHO), W. H. O. Cervical cancer. https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (2024).
-
- Ferlay, J. et al. Global cancer observatory: Cancer today. lyon, france: International agency for research on cancer. https://gco.iarc.who.int/today (2024).
MeSH terms
Substances
Grants and funding
- FRB660046/0162/the Fundamental Fund 2023 of Chiang Mai University
- FRB660046/0162/the Fundamental Fund 2023 of Chiang Mai University
- FRB660046/0162/the Fundamental Fund 2023 of Chiang Mai University
- FRB660046/0162/the Fundamental Fund 2023 of Chiang Mai University
- FRB660046/0162/the Fundamental Fund 2023 of Chiang Mai University
- FRB660046/0162/the Fundamental Fund 2023 of Chiang Mai University
- FRB660046/0162/the Fundamental Fund 2023 of Chiang Mai University
- FRB660046/0162/the Fundamental Fund 2023 of Chiang Mai University
- FRB660046/0162/the Fundamental Fund 2023 of Chiang Mai University
- FRB660046/0162/the Fundamental Fund 2023 of Chiang Mai University