Adipocyte-derived factors induce adherent to suspension transition in breast and pancreatic cancer cells through lipid metabolic alteration
- PMID: 40836054
- PMCID: PMC12368030
- DOI: 10.1038/s41598-025-13309-4
Adipocyte-derived factors induce adherent to suspension transition in breast and pancreatic cancer cells through lipid metabolic alteration
Abstract
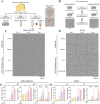
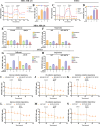
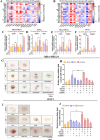
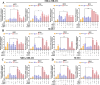
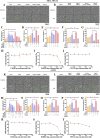
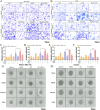
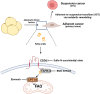
Adipocytes play a dynamic role in the tumor microenvironment (TME) by acting as facilitators, providing cytokines and metabolites that regulate cancer progression and metastasis. Despite metastasis being a major contributor to cancer-associated mortality, our understanding of how adipocytes influence this process remains limited. This study aims to elucidate the regulatory mechanism of Adherent to Suspension Transition (AST) reprogramming within the adipocyte, driven by anchorage dependency. AST facilitates the conversion of adherent tumor cells into suspension cells, thereby contributing to the generation of circulating tumor cells (CTCs). We have evaluated generating AST cells from primary tumors using a dissemination assay that mimics CTCs in vitro. Additionally, we examined AST cell formation when incubated with human adipocyte-conditioned media (ADCM) using the InCucyte live-cell imaging system. Through this approach, we effectively assessed the impact of the tumor-adipocyte interactions on CTC formation from the perspective of AST. As a metastasis-initiating marker, CD36 is pivotal in fatty acid (FA) acquisition and regulates lipid metabolic remodeling during the AST. The generation of AST cells through AST reprogramming is controlled by fatty acid oxidation (FAO), and pharmacological blockade of CD36 and FAO significantly reduced AST cell generation. This demonstrates that CD36 plays a key role in the early stages of AST-induced dissemination. Additionally, promoting cancer cell aggressiveness through ADCM enhances metastatic potency and upregulates the expression of AST reprogramming factors. Inhibition of lipid metabolism not only suppresses AST cell formation but also decreases survival in suspension. This indicates that exogenous lipid uptake and FAO via CD36 play crucial roles in the metastasis process, facilitating the dissemination of primary tumors into the bloodstream. Adipocytes contribute to cancer progression by supplying various metabolites to cancer cells. While primary tumors predominantly rely on glucose as a major energy source, cellular remodeling during dissemination shifts metabolic dependency toward lipids. In the TME, where adipocytes are abundant, tumor cells acquire FA through CD36-mediated uptake for metabolic adaptation. This shift to lipid metabolism is essential for AST, and thus, targeting lipid metabolism via inhibition of CD36 and FAO could serve as a potential therapeutic strategy for AST.
Keywords: AST; Adipocytes; CD36; Circulating tumor cell; Lipid metabolism; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Human adipose tissue usage received ethical approval from the Institutional Review Board at Gangnam Severance Hospital, Yonsei University, Seoul, Republic of Korea, adhering to the ethical principles outlined in the 1975 Declaration of Helsinki. Acquisition of human adipose tissue samples was conducted with the informed consent of participating patients, following guidelines and regulations approved by the Institutional Review Board of Gangnam Severance Hospital, Yonsei University Health System. Consent for publication: All authors have consented to their inclusion in the list and have approved the submission of this content. The material has not been presented for publication elsewhere during the review process for consideration. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Extracellular vesicles from ovarian cancer cells induce senescent lipid-laden macrophages to facilitate omental metastasis.J Nanobiotechnology. 2025 Jul 26;23(1):540. doi: 10.1186/s12951-025-03612-7. J Nanobiotechnology. 2025. PMID: 40713812 Free PMC article.
-
Identification of new selective CD36 inhibitors to potentiate HER2-targeted therapy in HER2-positive breast cancer.Sci Rep. 2025 Aug 6;15(1):28709. doi: 10.1038/s41598-025-14639-z. Sci Rep. 2025. PMID: 40770043 Free PMC article.
-
The evolving tumor-associated adipose tissue microenvironment in breast cancer: from cancer initiation to metastatic outgrowth.Clin Transl Oncol. 2025 Jul;27(7):2778-2788. doi: 10.1007/s12094-024-03831-8. Epub 2024 Dec 25. Clin Transl Oncol. 2025. PMID: 39720985 Review.
-
CD36: an emerging therapeutic target for cancer and its molecular mechanisms.J Cancer Res Clin Oncol. 2022 Jul;148(7):1551-1558. doi: 10.1007/s00432-022-03957-8. Epub 2022 Feb 27. J Cancer Res Clin Oncol. 2022. PMID: 35224665 Free PMC article. Review.
References
-
- Mukherjee, A., Bilecz, A. J. & Lengyel, E. The adipocyte microenvironment and cancer. Cancer Metastasis Rev.41, 575–587. 10.1007/s10555-022-10059-x (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

