Network synchrony creates neural filters promoting quiescence in Drosophila
- PMID: 40836080
- PMCID: PMC12527942
- DOI: 10.1038/s41586-025-09376-2
Network synchrony creates neural filters promoting quiescence in Drosophila
Abstract
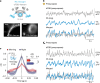
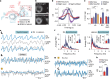
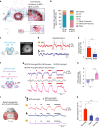
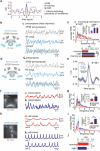
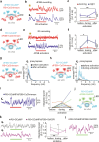
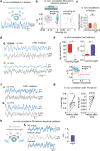
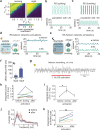
Animals require undisturbed periods of rest during which they undergo recuperative processes1. However, it is unclear how brain states arise that are able to dissociate an animal from its external world, enabling quiescent behaviours, while retaining vigilance to salient sensory cues2. Here we describe a neural mechanism in Drosophila that creates neural filters that engender a brain state that enables quiescent behaviour by generating coherent slow-wave activity (SWA)3 between sleep-need4 (R5)- and locomotion-promoting neural networks5. The coherence of SWA is subject to circadian and homeostatic control and can be modulated by sensory experience. Mimicry of coherent SWA reveals that R5 oscillations reduce responsiveness to visual stimuli by rhythmically associating neural activity of locomotion-promoting cells, effectively overruling their output. These networks can regulate behavioural responsiveness by providing antagonistic inputs to downstream head-direction cells6,7. Thus, coherent oscillations provide the mechanistic basis for a neural filter by temporally associating opposing signals, resulting in reduced functional connectivity between locomotion-gating and navigational networks. We propose that the temporal pattern of SWA provides the structure to create a 'breakable' filter, permitting the animal to enter a quiescent state, while providing the architecture for strong or salient stimuli to 'break' the neural interaction, consequently allowing the animal to react.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures









References
-
- Andrillon, T. & Kouider, S. The vigilant sleeper: neural mechanisms of sensory (de)coupling during sleep. Curr. Opin. Physiol.15, 47–59 (2020). - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

