Cancer-induced nerve injury promotes resistance to anti-PD-1 therapy
- PMID: 40836096
- PMCID: PMC12406299
- DOI: 10.1038/s41586-025-09370-8
Cancer-induced nerve injury promotes resistance to anti-PD-1 therapy
Abstract
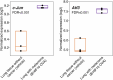
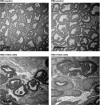
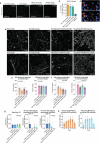
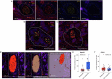
Perineural invasion (PNI) is a well-established factor of poor prognosis in multiple cancer types1, yet its mechanism remains unclear. Here we provide clinical and mechanistic insights into the role of PNI and cancer-induced nerve injury (CINI) in resistance to anti-PD-1 therapy. Our study demonstrates that PNI and CINI of tumour-associated nerves are associated with poor response to anti-PD-1 therapy among patients with cutaneous squamous cell carcinoma, melanoma and gastric cancer. Electron microscopy and electrical conduction analyses reveal that cancer cells degrade the nerve fibre myelin sheets. The injured neurons respond by autonomously initiating IL-6- and type I interferon-mediated inflammation to promote nerve healing and regeneration. As the tumour grows, the CINI burden increases, and its associated inflammation becomes chronic and skews the general immune tone within the tumour microenvironment into a suppressive and exhaustive state. The CINI-driven anti-PD-1 resistance can be reversed by targeting multiple steps in the CINI signalling process: denervating the tumour, conditional knockout of the transcription factor mediating the injury signal within neurons (Atf3), knockout of interferon-α receptor signalling (Ifnar1-/-) or by combining anti-PD-1 and anti-IL-6-receptor blockade. Our findings demonstrate the direct immunoregulatory roles of CINI and its therapeutic potential.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: K.Y.T. serves as a consultant to NFlection Therapeutics, Sun Pharma, DXB Biosciences. R.F. reports a consulting or advisory role at Regeneron, Sanofi, Elevar Therapeutics, Remix, Eisai, Bioatlas, Coherus in the past 24 months and research Funds (Inst) from Prelude, Ayala, Merck, Pfizer, Rakuten, EMD Serono, ISA, Viracta and Gilead in the past 24 months. N.D.G. reports institutional research funding from Regeneron and Ascendis Pharmaceuticals; and advisory board and consulting fees from PDS Biotechnology, Merck, Regeneron and GeoVax; royalties from UpToDate. M.A.D. has been a consultant to Replimmune, Nurix, Roche/Genentech, Array, Pfizer, Novartis, BMS, GSK, Sanofi-Aventis, Vaccinex, Apexigen, Eisai, Iovance, Merck and ABM Therapeutics, and has been the principal investigator of research grants to MD Anderson by Roche/Genentech, GSK, Sanofi-Aventis, Merck, Myriad, Oncothyreon, Pfizer, ABM Therapeutics and LEAD Pharma. N.J.D. received a reagent from Eterna Therapeutics, unrelated to the work presented here. J.A.W. is listed as an inventor on a US patent application (PCT/US17/53.717) submitted by the University of Texas MD Anderson Cancer Center that covers methods to enhance immune checkpoint blockade responses by modulating the microbiome; she reports compensation for speaker’s bureau and honoraria from PeerView and serves as a consultant and/or advisory board member for Gustave Roussy Cancer Center, OSE Immunotherapeutics, Bayer Therapeutics, James Cancer Center OSU and Daiichi Sankyo. N.I.K. is an advisory board member for Regeneron, Merck, Replimune, Immunocore, Iovance Biotherapeutics, Novartis, IO Biotech, MyCareGorithm and HUYABIO International; received travel support from Castle Biosciences and Regeneron; is a data safety monitoring board member for Incyte and AstraZeneca; a scientific advisory board member for T-Knife Therapeutics; a study steering committee member for BMS, Nektar, Regeneron and Replimune; holds common stock in Bellicum Pharmaceuticals and Amarin; and has received research funding (to institution) from BMS, Merck, Regeneron, Replimune, GSK, Celgene, Novartis, IDEAYA Biosciences, Modulation Therapeutics and HUYABIO International. P.S. is a scientific advisory committee member for Adaptive Biotechnologies, Akoya Biosciences, Apricity, Asher Bio, BioAtla, BioNTech, Catalio, C-Reveal Therapeutics, Dragonfly Therapeutics, Earli, Enable Medicine, Glympse, Henlius/Hengenix, Hummingbird, ImaginAb, InterVenn Biosciences, JSL Health, Lytix Biopharma, Marker Therapeutics, Matrisome, NTx, Oncolytics, Osteologic, PBM Capital, Phenomic Al, Polaris Pharma, Soley Therapeutics, Sporos, Time Bioventures, Two Bear Capital, Vironexis and Xilis; holds private investments in Affini-T, Candel Therapeutics, LAVA Therapeutics, Spotlight and Trained Therapeutix Discovery. N.D.G. reports institutional research funding from Regeneron Pharmaceuticals and Ascendis Pharma; speaker honoraria from AiCME and OncLive; and is an advisory board member and received consulting fees from PDS Biotechnology, Regeneron Pharmaceuticals, Merck and GeoVax. G.A.C. is one of the scientific founders of Ithax Pharmaceuticals. C.H. declares no competing interests related to ‘A Randomized Phase II Trial of Induction Pembrolizumab Followed by Surgery Versus Surgery Alone for Resectable Cutaneous Squamous Cell Carcinoma’. M.K.W. has participated in advisory boards for Regeneron, Castle Biosciences, Replimune, Incyte and Sun Pharma. M.R.M. is a paid advisor for Feldan, Regeneron Pharmaceuticals, Replimune, Philogen, Sanofi, Stamford Pharmaceuticals and Sun Pharmaceuticals; and received research funding from Regeneron Pharmaceuticals, Replimune, Sanofi, Solgel and Senhwa. K. Rai has equity in Jivanu Therapeutics and Koshika Therapeutics. The other authors declare no competing interests.
Figures










Update of
-
Inflammation induced by tumor-associated nerves promotes resistance to anti-PD-1 therapy in cancer patients and is targetable by interleukin-6 blockade.Res Sq [Preprint]. 2023 Jul 18:rs.3.rs-3161761. doi: 10.21203/rs.3.rs-3161761/v1. Res Sq. 2023. Update in: Nature. 2025 Oct;646(8084):462-473. doi: 10.1038/s41586-025-09370-8. PMID: 37503252 Free PMC article. Updated. Preprint.
References
-
- Liebig, C., Ayala, G., Wilks, J. A., Berger, D. H. & Albo, D. Perineural invasion in cancer. Cancer115, 3379–3391 (2009). - PubMed
-
- Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med.372, 2521–2532 (2015). - PubMed
-
- Seiwert, T. Y. et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol.17, 956–965 (2016). - PubMed
MeSH terms
Substances
Grants and funding
- RM1 DE033491/DE/NIDCR NIH HHS/United States
- UH3 TR000943/TR/NCATS NIH HHS/United States
- K08 CA270417/CA/NCI NIH HHS/United States
- P30 CA076292/CA/NCI NIH HHS/United States
- U54 CA096300/CA/NCI NIH HHS/United States
- R01 CA200263/CA/NCI NIH HHS/United States
- R01 CA182905/CA/NCI NIH HHS/United States
- P50 CA127001/CA/NCI NIH HHS/United States
- R01 CA272946/CA/NCI NIH HHS/United States
- R01 DE032501/DE/NIDCR NIH HHS/United States
- R01 CA250214/CA/NCI NIH HHS/United States
- R01 DE029493/DE/NIDCR NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- R01 CA222007/CA/NCI NIH HHS/United States
- R01 DE033674/DE/NIDCR NIH HHS/United States
- U54 CA096297/CA/NCI NIH HHS/United States
- R01 DE032018/DE/NIDCR NIH HHS/United States
- R01 CA266529/CA/NCI NIH HHS/United States
- R01 DE032712/DE/NIDCR NIH HHS/United States
- R01 GM122775/GM/NIGMS NIH HHS/United States
- R37 CA242006/CA/NCI NIH HHS/United States
- R50 CA243707/CA/NCI NIH HHS/United States
- P50 CA221703/CA/NCI NIH HHS/United States
- R01 AI133822/AI/NIAID NIH HHS/United States
- R35 DE027551/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

