Hypoxia-induced exosomal circNRIP1 activates cancer-associated fibroblasts to promote esophageal squamous cell carcinoma migration and invasion
- PMID: 40836210
- PMCID: PMC12366159
- DOI: 10.1186/s12876-025-03978-w
Hypoxia-induced exosomal circNRIP1 activates cancer-associated fibroblasts to promote esophageal squamous cell carcinoma migration and invasion
Abstract
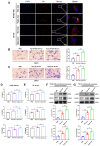
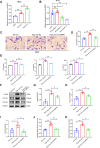
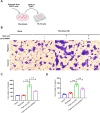
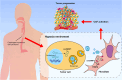
Esophageal squamous cell carcinoma (ESCC) is characterized by a complex tumor microenvironment (TME). Cancer-associated fibroblasts (CAFs) play a crucial role in the TME that facilitate tumor progression via interactions with cancer cells. However, the mechanisms underlying the activation of CAFs in TME remain largely unknown. Here, we characterized the exosomes derived from normoxic and hypoxic ESCC cells using electron microscopy and western blot. The impact of exosomes on CAF activation and the motility of ESCC cells was examined in vitro. The molecular complex involving circNRIP1 was explored using RNA pull-down. We demonstrated that exosomes derived from ESCC cells, including KYSE-150 and TE-10 cells, exhibited a significantly increase in secretion under hypoxic conditions. These hypoxic exosomes were internalized by fibroblasts and further promoted the transformation of normal fibroblasts into CAFs, as evidenced by enhanced migration and secretion of pro-inflammatory cytokines. circNRIP1 was enriched in hypoxic exosomes, and its absence abolished the effect of hypoxic exosomes to activate CAFs. Furthermore, the CAFs activated by exosomal circNRIP1 further promoted the migration and invasion of ESCC cells. Mechanistically, circNRIP1 bound to the N1-methyladenosine (m1A) methyltransferase TRMT6 and activated CAFs in a TRMT6-dependent manner. This study revealed the role of hypoxia-induced exosomal circNRIP1 in the activation of CAFs, which contributes to ESCC development. These findings shed light on the mechanisms of the CAF activation in ESCC, positioning hypoxia-induced exosomal circNRIP1 as a potential molecular target for ESCC.
Keywords: Cancer-associated fibroblast; CircNRIP1; Esophageal squamous cell carcinoma; Exosome; Hypoxia.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Morgan E, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: New estimates from GLOBOCAN 2020. J Gastroenterol. 2022;163(3):649–658.e2. - PubMed
-
- Thrift AP. Global burden and epidemiology of Barrett oesophagus and oesophageal cancer. J Nat Rev Gastroenterol Hepatol. 2021;18(6). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

