Comparative cardiovascular outcomes and safety of hypoglycemic drug classes in patients with type 2 diabetes and hypertension: a multicenter cohort analysis
- PMID: 40836299
- PMCID: PMC12369064
- DOI: 10.1186/s12933-025-02892-5
Comparative cardiovascular outcomes and safety of hypoglycemic drug classes in patients with type 2 diabetes and hypertension: a multicenter cohort analysis
Abstract
Background: Patients with type 2 diabetes (T2D) and hypertension are at increased risk of adverse cardiovascular (CV) events. However, real-world evidence comparing the CV effectiveness and safety of major hypoglycemic drug classes remains limited in this population. This multicenter pooled analysis aims to directly compare the CV outcomes and safety profiles of these key agents in patients with T2D and hypertension.
Methods: We analyzed electronic health records from two databases in a cohort study of T2D patients with hypertension who had initiated metformin as first-line therapy. Propensity score matching (PSM) and Cox proportional hazards models were used to compare the risks of 3-/4-point major adverse cardiovascular events (MACE) and safety outcomes across drug classes added to metformin: insulin, sulfonylureas (SUs), glucagon-like peptide-1 receptor agonists (GLP-1 RAs), dipeptidyl peptidase-4 inhibitors (DPP4is), glinides, acarbose, and sodium-glucose transporter 2 inhibitors (SGLT2is).
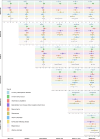
Results: Compared with insulin, GLP-1 RAs, DPP4is, and glinides were associated with a lower risk of 3-point MACE (HR: 0.48 [0.31-0.76], 0.70 [0.57-0.85], and 0.70 [0.52-0.94], respectively). SUs were associated with a higher risk of 3-point MACE compared with DPP4is (HR: 1.30 [1.06-1.59]). DPP4is, GLP-1 RAs, and glinides showed a lower risk of 3-point MACE compared with acarbose (HR: 0.62 [0.51-0.76], 0.47 [0.29-0.75], and 0.59 [0.43-0.81], respectively). Similar patterns were observed for 4-point MACE. For safety outcomes, DPP4is were associated with a reduced risk of chronic kidney disease, while insulin use was associated with reduced risks of inflammatory polyarthritis and insomnia. However, DPP4is were associated with higher risks of coronary atherosclerotic diseases and hypertensive heart disease.
Conclusions: This study highlights the differential cardiovascular effectiveness and safety profiles of hypoglycemic therapies in real-world settings, providing valuable insights for optimizing T2D management, particularly in patients with comorbid hypertension.
Keywords: Cardiovascular safety; Hypertension; Major adverse cardiovascular events (MACE); Real-world evidence; Retrospective study; Type 2 diabetes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Ethics Committee of Nanjing Medical University (2023(498)), with informed consent waived due to its retrospective nature. It adhered to the Declaration of Helsinki and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. Competing interests: The authors declare no competing interests.
Figures


References
-
- Bi Y, Li M, Liu Y, Li T, Lu J, Duan P, et al. Intensive blood-pressure control in patients with type 2 diabetes. N Engl J Med. 2024;392(12):1155–67. - PubMed
-
- Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–30. - PubMed
-
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82404291/National Natural Science Foundation of China (NSFC) Youth Science Fund
- BK20241308/Basic Research Program of Jiangsu Province
- 2024ZD0531800/National Science and Technology Major Project
- 2023YFC3605800/National Key Research and Development Program of China
- QY202403/Leading Major Projects in Basic Research at the Forefront of the Institute (China)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

