The toxic effect of titanium dioxide nanoparticles on rat submandibular salivary glands and the protective role of vitamin E
- PMID: 40836331
- PMCID: PMC12366081
- DOI: 10.1186/s12903-025-06631-w
The toxic effect of titanium dioxide nanoparticles on rat submandibular salivary glands and the protective role of vitamin E
Abstract
Background: To assess the impact of titanium dioxide nanoparticles (TiO2NPs) on submandibular salivary glands and the role of vitamin E in preventing this cytotoxicity.
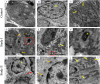
Methods: Thirty adult male albino rats were randomly divided into 3 groups: Negative control received olive oil for 3 weeks; Study I received olive oil for 1 week, then daily oral administration of 300 mg/kg TiO2NPs for 2 weeks; and Study II received 100 mg/kg vitamin E diluted in 100 ml olive oil daily as a prophylactic from day 1 for 3 weeks. On day 8, with vitamin E, they received 300 mg/kg TiO2NPs for 2 weeks by oral gavage. All samples were examined via hematoxylin & eosin (H&E), histomorphometry of serous acinar surface areas, transmission electron microscopy (TEM), and blood analysis of malondialdehyde (MDA) and interleukin (IL-1β) levels.
Results: Serum levels of both MDA and IL-1β were significantly greater in study I than in control and study II groups. Histologic examination revealed structural changes in serous acini and ducts of study I, with great preservation of the normal appearance of the acini and ducts in study II. Histomorphometry revealed a significant difference between control and study I, with no significant difference from that in study II. TEM revealed multiple ultrastructural changes in acinar cells and ducts of study I compared with those of study II, which maintained their normal features.
Conclusions: Vitamin E plays crucial antioxidant and anti-inflammatory roles in counteracting the cytotoxic effects of TiO2NPs by alleviating their deleterious impact on salivary glands.
Keywords: Nanoparticles; Nanotechnology; Submandibular glands; TiO2; Vitamin E.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: The Research Ethics Committee, Faculty of Dentistry, Alexandria University, approved the current study IRB: 0678-5/2023. The current experiment complies with the ARRIVE guidelines, EU Directive 2010/63/EU for animal experiments and the National Research Council’s Guide for the Care and Use of Laboratory Animals [56]. Informed consent: Not applicable. Clinical trial number: not applicable.
Figures




References
-
- Abdel Aal SM, Ahmed SM, Abdelrahman SA, Abdelrahman AA, Samy W. Duration-dependent effects induced by titanium dioxide nanoparticles on pancreas of adult male albino rats (histological and biochemical study). Ultrastruct Pathol. 2020;44:342–58. 10.1080/01913123.2020.1786203. - PubMed
-
- Chen Z, Han S, Zheng P, Zhou D, Zhou S, Jia G. Effect of oral exposure to titanium dioxide nanoparticles on lipid metabolism in Sprague–Dawley rats. Nanoscale. 2020;12:5973–86. 10.1039/C9NR10947A. - PubMed
-
- Stefaniak A, Hoover M, Nanotechnology N. Occupational Exposure to Titanium Dioxide [Internet]. Available from: www.cdc.gov/niosh
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

