The down-regulation of MsWOX13-2 promotes enhanced waterlogging resilience in alfalfa
- PMID: 40836384
- PMCID: PMC12368323
- DOI: 10.1111/tpj.70411
The down-regulation of MsWOX13-2 promotes enhanced waterlogging resilience in alfalfa
Abstract
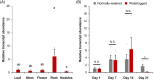
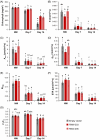
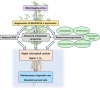
Soil waterlogging events are predicted to escalate globally as a result of climate change, threatening the sustainability of alfalfa (Medicago sativa L.) and livestock production in the future. WUSCHEL-related homeobox (WOX) transcription factors are known to play a role in numerous developmental processes and abiotic stress responses; however, their function in waterlogging resilience has not been investigated as of yet. In the present study, we functionally characterized the alfalfa MsWOX13-2 gene, which we found to be differentially expressed in response to waterlogging. Although the RNAi-mediated silencing of MsWOX13-2 in alfalfa did not affect growth or morphology under normally watered conditions, MsWOX13-2 RNAi plants exhibited higher chlorophyll retention and maximum quantum efficiency of photosystem II, as well as greater survivability, compared to empty vector genotypes under waterlogging. Subsequent analyses indicated that MsWOX13-2 RNAi leaves accumulated less H2O2 and displayed a greater increase in superoxide dismutase activity under waterlogging, resulting in reduced oxidative damage, which may have contributed to the enhanced waterlogging tolerance in these genotypes. RNA-Seq analysis confirmed alterations in the transcript levels of genes related to antioxidants, as well as those involved in photosynthesis, anaerobic fermentation, phytohormone-related pathways, and transcriptional regulation in the leaves of WOX13-2 RNAi genotypes compared to wild type following waterlogging stress. Bi-allelic mutation of MsWOX13-2 in alfalfa using CRISPR/Cas9 confirmed its function in waterlogging response. Overall, our findings suggest that MsWOX13-2 acts as a negative regulator of waterlogging response in alfalfa, providing a novel candidate for downstream breeding endeavors in this important species.
Keywords: Medicago sativa; MsWOX13‐2; alfalfa; crop improvement; forage; waterlogging tolerance.
© 2025 His Majesty the King in Right of Canada and The Author(s). The Plant Journal published by Society for Experimental Biology and John Wiley & Sons Ltd. Reproduced with the permission of the Minister of Agriculture and Agri‐Food Canada.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Ahsan, N. , Lee, D.G. , Lee, S.H. , Kang, K.Y. , Bahk, J.D. , Choi, M.S. et al. (2007) A comparative proteomic analysis of tomato leaves in response to waterlogging stress. Physiologia Plantarum, 131, 555–570. - PubMed
-
- Anjum, N.A. , Sharma, P. , Gill, S.S. , Hasanuzzaman, M. , Khan, E.A. , Kachhap, K. et al. (2016) Catalase and ascorbate peroxidase—representative H2O2‐detoxifying heme enzymes in plants. Environmental Science and Pollution Research, 23, 19002–19029. - PubMed
-
- Arbona, V. , Hossain, Z. , Lopez‐Climent, M.F. , Perez‐Clemente, R.M. & Gomez‐Cadena, A. (2008) Antioxidant enzymatic activity is linked to waterlogging stress tolerance in citrus. Physiologia Plantarum, 132, 452–466. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

